Ketogenic Diets Cause Cellular Senescence in Mice
- Fat saturation did not seem to matter in this experiment.

A new study tested two ketogenic diets and found increases in cellular senescence in multiple tissues [1].
It’s complicated
Ketogenic diets have been a matter of substantial debate. On one hand, they are effective against childhood epilepsy [2], have shown some promise against neurodegeneration [3] and as an adjuvant anti-cancer therapy [4], and might help people lose weight. Anecdotally, people have reported increased energy levels and other positive effects from ketogenic diets, but rigorous studies in humans are still scarce.
On the other hand, ketogenic diets have been linked to increased levels of harmful LDL cholesterol [5], a major factor in cardiovascular disease. A ketogenic diet in epilepsy patients has been associated with increased prevalence of kidney stones and bone fractures [6]. In general, long-term effects of ketogenic diets are not well understood, which gives many experts pause.
In this new study published in Science Advances, researchers from the University of Texas investigated the effects of two ketogenic diets on cellular senescence in mice, reporting intriguing results.
A spike in senescence
The two ketogenic diets (KDs) had vastly different ratios of saturated versus unsaturated fats but produced largely similar results. In both diets, almost none of the calories came from carbohydrates: around 10% came from protein, and around 90% came from fat. Importantly, these are proper ketogenic diets, even if a bit extreme, and they differ from the high-fat diets used by scientists to cause weight gain and metabolic dysfunction in mice, in which only about 40-50% of calories come from fat.
The controls in this study received a balanced diet with most of the calories coming from carbohydrates, and some from protein and fat. Mice in all the groups consumed virtually the same number of calories, and no significant weight gain in any group was reported, although a slight increase in body weight was observed in mice on KDs after 21 days.
At the end of this period, the researchers saw sharp increases in the most popular marker of cellular senescence, senescence-associated beta-galactosidase (SA-β-gal), and in two other markers, histone protein macroH2A.1 (H2AY) and histone 3 lysine 9 trimethylation (H3K9me3), in liver and kidney tissue. Substantial increases in two additional markers, p21 and p16, were detected in four tissues: heart, kidney, liver, and brain.
The prevalence of senescence seemed to be high, with 15% to 20% of the cells in the heart and 10% to 15% of the cells in the kidney stained positive for SA-β-gal. The researchers note that similar levels of senescent cell burden have been reported in murine models of myocardial infarction, lung cell damage, and radiation exposure.
In line with previous research, mice on KDs showed signs of metabolic dysregulation, with some deficit in glucose uptake, but no changes in insulin sensitivity. Levels of triglycerides, LDL cholesterol, and HDL cholesterol were elevated after 21 days.
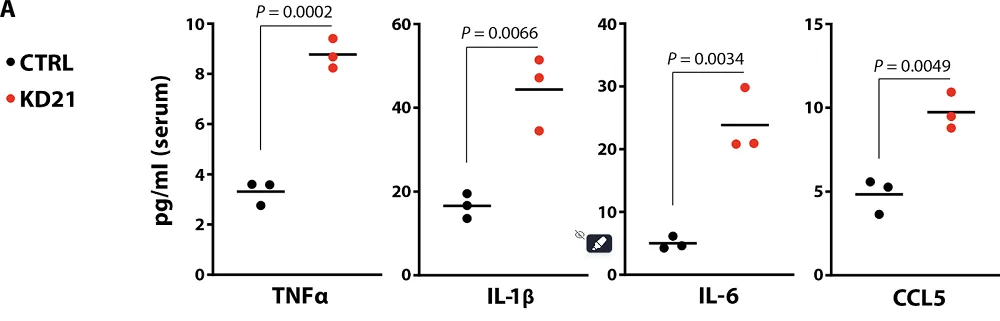
Senescent cells emit the senescence-associated secretory phenotype (SASP), a mix of various factors that have been reported to contribute to inflammation and induce senescence in nearby cells. The researchers analyzed several pro-inflammatory SASP molecules, TNFα, IL-1β, IL-6, and CCL5, and found significantly elevated levels of them in mice after 21 days of KD:
Similar data from human plasma
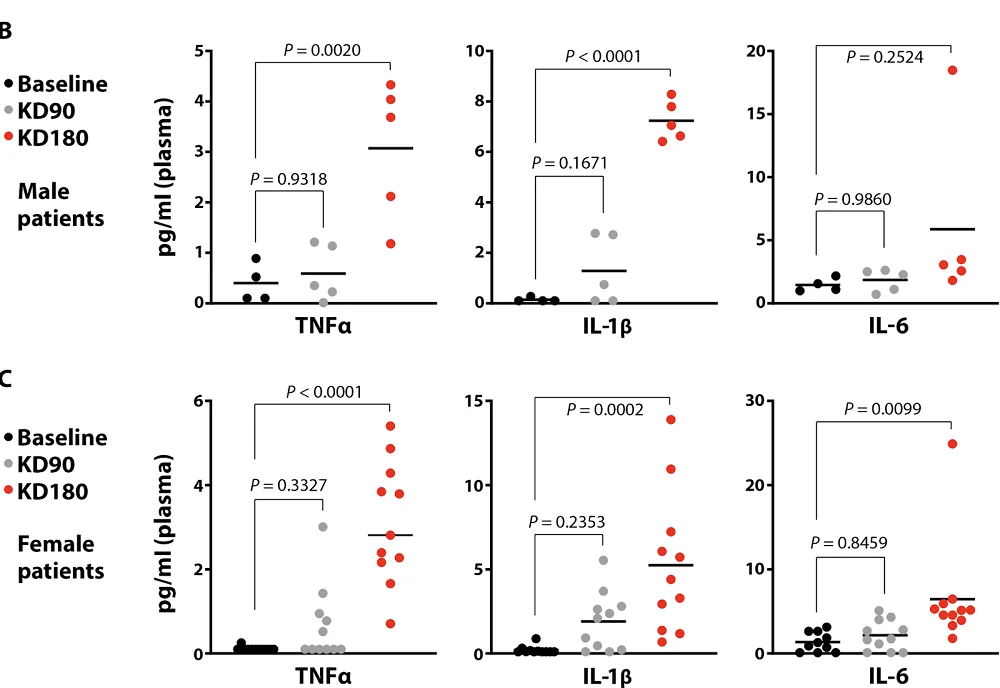
The group also analyzed data from two human KD trials at the University of Texas and received similar results. Large increases in two SASP factors (TNFα and IL-1β) in human plasma were detected in both males and females. Interestingly, the increases were slight and statistically insignificant after 90 days of a KD but much more pronounced after 180 days. These results might add to the uneasiness about the long-term effects of KDs. Many interventional studies of KDs in humans are of shorter duration and might have overlooked these effects.
Putting the mice back on regular diets led to a gradual return of the senescence markers to their normal values. According to the researchers, this suggests that KD-related cellular senescence is reversible, at least up to a certain point, and senescent cells get cleared away once the senescence-inducing effects of a KD are no more. The group also found that the negative effects of KDs they had discovered are age-independent, occurring in both young and aged mice.
The results of our in vivo murine experiments in this study, as well as those from other laboratories, reinforce that the effects of KD are complex, with both potential benefits and side effects likely due to multiple factors, including the timing, composition of the diet and the genetics, endocrine factors, and health conditions of the individual. As such, it is proposed that the use of a KD should be considered within the overall scope of personalized medicine, in which the variables for each patient are taken into consideration to determine who will, and who will not, benefit from this dietary intervention or a specific regimen.
Literature
[1] Wei, S. J., Schell, J. R., Chocron, E. S., Varmazyad, M., Xu, G., Chen, W. H., … & Gius, D. (2024). Ketogenic diet induces p53-dependent cellular senescence in multiple organs. Science Advances, 10(20), eado1463.
[2] Neal, E. G., Chaffe, H., Schwartz, R. H., Lawson, M. S., Edwards, N., Fitzsimmons, G., … & Cross, J. H. (2008). The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. The Lancet Neurology, 7(6), 500-506.
[3] Pinto, A., Bonucci, A., Maggi, E., Corsi, M., & Businaro, R. (2018). Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer’s disease. Antioxidants, 7(5), 63.
[4] Allen, B. G., Bhatia, S. K., Anderson, C. M., Eichenberger-Gilmore, J. M., Sibenaller, Z. A., Mapuskar, K. A., … & Fath, M. A. (2014). Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox biology, 2, 963-970.
[5] Iatan, I., Huang, K., Vikulova, D., Ranjan, S., & Brunham, L. R. (2024). Association of a low-carbohydrate high-fat diet with plasma lipid levels and cardiovascular risk. JACC: Advances, 100924.
[6] Groesbeck, D. K., Bluml, R. M., & Kossoff, E. H. (2006). Long-term use of the ketogenic diet in the treatment of epilepsy. Developmental medicine and child neurology, 48(12), 978-981.