Netrin-1 Rescues Blood Stem Cells in Mice
- This protein appears to be critical for the cell cycle and DNA repair.
Scientists have discovered that the protein Netrin-1 alleviates the age-related decline in hematopoietic stem cell function in mice, enhancing HSC transplantation and protecting these mice from the harmful effects of chemotherapy [1].
(Not) a niche problem
Declining stem cell function (stem cell exhaustion) is linked to numerous aging phenotypes [2]. Stem cells reside in so-called “niches” – microenvironments whose role is to support stem cells’ viability and function. However, stem cell niches themselves are prone to accumulating age-related damage [3].
In this new study, the researchers “sought to determine whether aged blood stem cell function can be restored by rejuvenating their supportive niches within the bone marrow”. The health of hematopoietic stem cells, which give rise to differentiated blood cells, is especially important in the context of blood cancer, with patients often requiring hematopoietic stem cell transplantation (HSCT). HSCT effectiveness diminishes with age, probably due to age-related changes in the bone marrow niche, although the exact mechanisms of this deterioration remain unknown [4].
Target: Netrin-1
The researchers used a mouse model that recapitulates premature aging of the immune system. Transcriptomic analysis revealed multiple genes that were differentially expressed in this model compared to wild-type mice, and the researchers focused on the one that encodes Netrin-1. Since this protein has been linked to angiogenesis (blood vessel building) and osteogenesis (bone building), it seemed plausible that it might be relevant to the BM stem cell niche.
Perusing existing RNA sequencing databases revealed that in bone marrow, Netrin-1 is expressed mostly in mesenchymal stromal cells (MSCs) and epithelial cells (ECs), both of which are important components of the niche. Conditional deletion of Netrin-1 in those cells led to impaired vascular integrity and accumulation of adipocytes (fat cells), both of which are known features of BM niche aging.
More importantly, Netrin-1 deletion seemed to impair stem cell function. In mice with Netrin-1-deficient MSCs, more HSCs were “stuck” in the nonproliferative G0 phase of the cell cycle. This decrease in progenitor activity was accompanied by impaired engraftment capacity when HSCs from Netrin-1-deficient mice were transplanted into wild-type mice. In mice with Netrin-1 knocked down in ECs, similar effects were observed, albeit of a lesser magnitude. An increase in the number of quiescent stem cells and a decline in their engraftment capacity are characteristic of hematopoietic aging.
DNA damage responses restored
The researchers performed RNA sequencing to elucidate the effect of Netrin-1 deletion on gene expression. In MSCs and ECs of Netrin-1-deficient mice, they detected significant upregulation of pathways associated with adipogenesis, cell cycle, and, importantly, DNA damage responses (DDR). Consequently, those cells accumulated more DNA damage than controls.
Accumulation of DNA damage is central to cellular aging. In this experiment, DNA damage was greater in MSCs and ECs taken from aged wild-type mice than those taken from their young counterparts. Strikingly, a two-week Netrin-1 treatment was enough to significantly reverse DNA damage in aged mice. The treatment also resulted in improved bone marrow vascular health.
Protection from chemotherapy-induced damage
However, the important question was whether Netrin-1 supplementation would improve the function of an aged hematopoietic system, which it did. The treatment increased the number of functional HSCs following bone marrow transplantation four-fold. Moreover, HSCs from treated aged mice were almost as fit and competent as those derived from young controls.
The researchers also learned that not all beneficial effects of Netrin-1 supplementation on HSCs result from improvements in the bone marrow niche. Many of those effects were recapitulated when HSCs were co-cultured with Netrin-1 in vitro, showing that Netrin-1 probably exerts its benefits both directly and indirectly, via niche improvements.
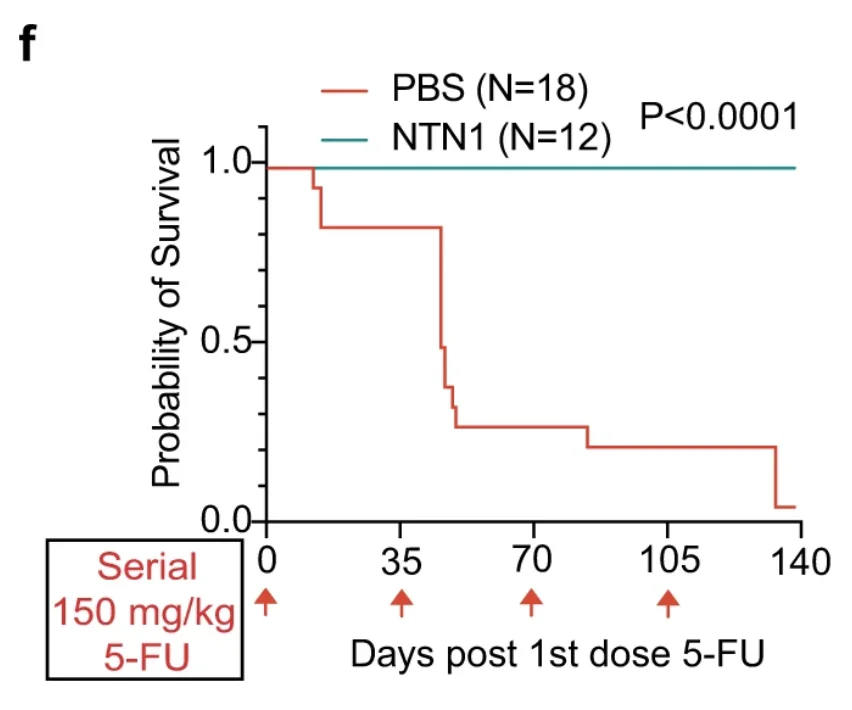
Netrin-1 treatment also significantly protected mice from the detrimental effects of chemotherapy. Treated mice demonstrated preservation of body weight and much more robust hematopoietic recovery. When mice were subjected to an especially damaging multiple-dose chemotherapy, the difference in survival was striking, with the treatment group not losing a single mouse:
Conclusion
This study identifies Netrin-1 as a regulator of BM niche health and HSC fitness that at least partially works by restoring DNA damage responses. This discovery might prove important for protecting patients from the harms of chemotherapy and improving HSC transplantation, and it might also apply to the wider context of aging.
Literature
[1] Ramalingam, P., Gutkin, M. C., Poulos, M. G., Tillery, T., Doughty, C., Winiarski, A., … & Butler, J. M. (2023). Restoring bone marrow niche function rejuvenates aged hematopoietic stem cells by reactivating the DNA Damage Response. Nature Communications, 14(1), 2018.
[2] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[3] Brunet, A., Goodell, M. A., & Rando, T. A. (2022). Ageing and rejuvenation of tissue stem cells and their niches. Nature Reviews Molecular Cell Biology, 1-18.
[4] Ho, Y. H., & Méndez-Ferrer, S. (2020). Microenvironmental contributions to hematopoietic stem cell aging. Haematologica, 105(1), 38.