“Everything Happens in Berlin”: Highlights From The Summit
- Many biotech companies promoted novel approaches.

The Rejuvenation Startup Summit in Berlin might not be the biggest or the longest-running longevity conference, but it never disappoints. We present you with the highlights from this vibrant, biotech-oriented event. As always, our apologies go to those equally worthy speakers who did not make the cut.
A longevity city in the making
The Rejuvenation Startup Summit in Berlin, hosted by Michael Greve’s Forever Healthy Foundation, is regarded as one of the most important longevity events of the year for a reason. Despite spanning “only” two days, the summit is always packed with action, with an important emphasis, as its name suggests, on longevity biotech rather than on academic research. Longevity biotech is a newborn field that is still small, frail, and full of risks and uncertainties, and it is crucial for people working in it to coordinate their efforts and support each other if we want to see rejuvenation therapies mature soon.

This time, however, the two conference days were just the crown jewel of the Longevity Week Berlin. The latter kicked off on May 6th with an opening event co-anchored by longevity advocate Andrew Steele and Lifespan.io executive director Stephanie Dainow. The evening included a well-appointed panel on democratizing longevity and a talk by Dr. Ina Czyborra, Berlin senator for science, health, and care, who told the audience about the city’s vast plan to build a longevity-friendly infrastructure for the benefit of its entire population. Many speakers noted that Berlin is quickly becoming one of the most future-oriented and startup-friendly cities in Europe, or, to quote one speaker, “Everything happens in Berlin.”
Longevity Week’s other events were self-organized and at times quirky, with titles such as “Longevity meets Nightlife” and “Longevity x Psychedelics: how trauma impacts aging and psychedelics could heal.” Meetups and industry lunches were abundant, and Google’s Berlin office hosted a dedicated event on longevity startups.
The Hevolutionary advantage
All this, however, was only a lead-up to the main course. The Summit itself commenced on May 10th with a keynote talk by Mehmood Khan, CEO of Hevolution, the Saudi non-profit fund that has been pouring considerable money into longevity research. Khan brings to the longevity field decades of experience managing giant companies such as PepsiCo, and his presentation skills are always on full display at conferences. Khan is also an MD with a background in endocrinology.
Khan started with the overview of the challenges with which “population greying” is presenting the world. Even the wealthiest countries are beginning to feel the burden of caring for their elderly, most of whom spend their last decade in poor health, constantly requiring costly treatments. According to Khan, this problem might be even more severe in developing countries which also experience populational aging but lack the resources and the infrastructure for adequate care.
Hevolution wants to change this situation by working across the “longevity ecosystem.” According to Khan, all the links in this chain are crucial to success. Interestingly, several speakers at the conference suggested that approved longevity medicines are a prerequisite for igniting the public’s interest. However, there is an alternative point of view: that shifts in public opinion should come first, driving a subsequent dramatic increase in funding, as was the case with the War on Cancer in the last century.
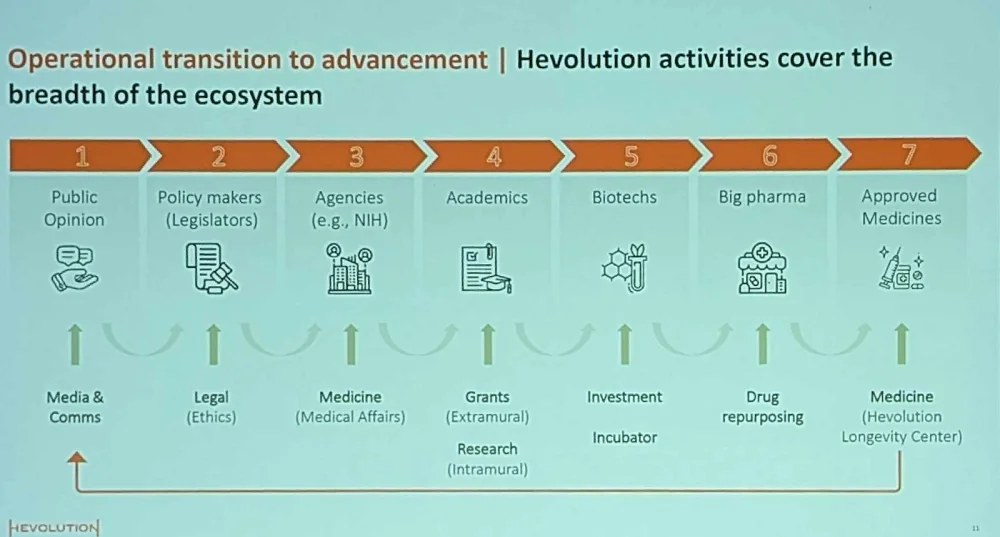
Hevolution, it seems, subscribes to the first view: its activities are largely focused on funding research, with about 250 million dollars committed to date. As an advocacy group, Lifespan.io leans towards the competing idea, which assigns vital importance to recruiting public opinion and influencing decision making in order to generate even more progress.
Going after “zombie cells”
Senolytics had a bumpy start in longevity. Several startups quickly moved into clinical trials, and then one of the industry’s first high-profile flops happened, when UNITY’s candidate drug failed Phase 2 trials. Still, many companies believe in senolytics, and they were abundantly represented at the conference.
Otto Kanzler of Rockfish Bio recounted the drawbacks that plague the first generation of senolytics. Drugs based on anti-apoptotic pathways, he said, have low selectivity and, consequently, harsh side effects. On top of that, there is still a lack of non-invasive clinical biomarkers for senescent cell load and a problem of choice between dozens of possible indications. Hence, new approaches are needed to unleash senolytics’ full potential.
Kanzler’s company identified a novel pathway based on phospholipase A2 (PLA2), an enzyme that shows increased activity in senescent cells. PLA2 facilitates apoptosis, a type of cellular death that senescent cells are famous for evading. How do these two facts square with each other? Apparently, senescent cells actively convert PLA2 before it can trigger apoptosis. Rockfish Bio’s lead candidate drug targets this conversion, helping apoptosis go through in senescent cells without harming healthy cells.
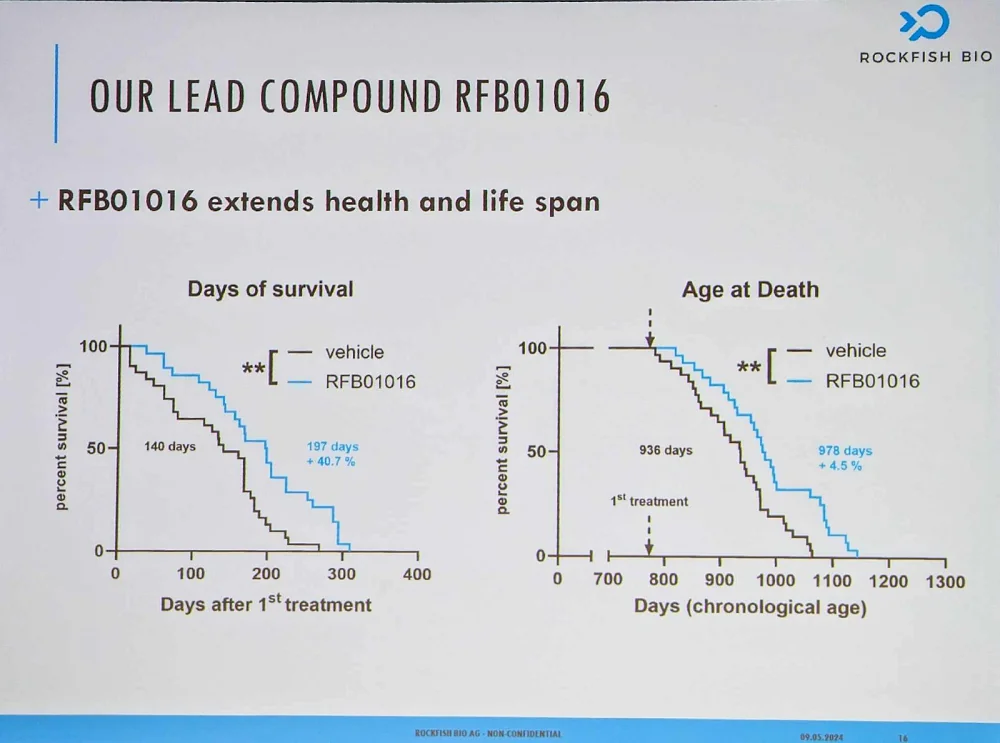
Kanzler reported encouraging efficacy data from mouse trials. With diminished senescent cell burden, mice showed less frailty and extended lifespan even though the treatment had been administered late in life.
The company’s strategy is to gain accelerated approval against a rare disease – probably idiopathic pulmonary fibrosis (IPF), a deadly disease tightly linked to cellular senescence – and then promote off-label use for other indications. Rockfish Bio also boasts its own SenomiR diagnostic tool, which can quantify senescent cell burden. In the company’s vision, older people will be undergoing the test routinely, and, if found eligible, will receive a senolytic therapy.
Marco Quarta of Rubedo spoke next. Rubedo is a well-established senolytic startup that recently received generous funding from Hevolution. The company employs a proprietary drug discovery platform, Alembic, which uses single-cell multi-omics to selectively target the very cells that drive pathology in various age-related diseases. The company’s lead program tackles atopic dermatitis and psoriasis. These might not be the fanciest targets, but Rubedo, like many other companies, aims to gain approval for existing indications and then promote their drugs’ off-label use in a more anti-aging context.
Like Kanzler before him, Quarta lamented the lack of selectivity and safety of first generation senolytics. He hopes, however, that Alembic can overcome this problem by identifying molecules with a more targeted action. As usual, the problem lies in the high heterogeneity of senescent cells across cell types and tissues. Meanwhile, Rubedo’s lead candidate has shown efficacy and was well-tolerated in a mouse model of chronic atopic dermatitis, raising hopes that it can become one of the first approved senolytics.
Eric Verdin, head of the Buck Institute for Research on Aging and one of the few speakers not associated with a particular biotech company, gave an overview of the Buck’s research activities, focusing on cellular senescence. Given the heterogeneity of senescence, characterization of senescent cells is of utmost importance. Researchers at the Buck have been studying gene expression changes in immune cells positive for various senescence markers, mainly β-galactosidase. They also found that senescent cells can be especially abundant (up to 40%) in some immune cell populations. Since immune cells travel across the body, they probably induce secondary (paracrine) senescence in various tissues.
Verdin discussed two more novel markers of senescence. One is chromatin fragmentation. Apparently, during senescence, the chromatin in the nucleus gets excised and translocates to the cytoplasm. “These cells obviously have massive DNA damage, which is not reversible”, Verdin said, adding that this poses serious questions about the feasibility of the senomorphic approach, which seeks to “fix” senescent cells instead of eliminating them.
Verdin also talked about lipofuscin, a yellow-brown pigment composed of lipid-containing residues which accumulate due to lysosomal stress. Lipofuscin is obviously linked to aging, but Verdin sees it as a good marker of specifically cellular senescence, at least in immune cells. Solving cellular senescence in skin and immune cells, according to Verdin, will also go a long way in getting rid of lipofuscin.
Lorna Harries of SENISCA, a company spun out of Harries’ academic team, is trying to do specifically what Eric Verdin warns about: restore the function of senescent cells rather than get rid of them. By using oligonucleotides for precision alteration of gene expression, SENISCA goes after another novel marker of senescence, which they also consider a new hallmark of aging: dysregulated RNA splicing.
According to Lorna, dysregulated splicing affects numerous aspects of cellular function, such as telomere maintenance and mRNAs coding for senescence-associated secretary phenotype (SASP) proteins, the mix of molecules emitted by senescent cells. She calls fixing senescent cells with oligonucleotides “reprogramming”, which is “analogous to the sort of reprogramming you would see with the Yamanaka factors, but without the changes in cell identity.” Under this treatment, Lorna says, senescent cells not just stop being harmful but can regain their original function.
Similarly to Rockfish Bio, the top indication that SENISCA is working on is idiopathic pulmonary fibrosis. In vitro, in cells derived from patients, the treatment led to reductions in senescence, DNA damage, inflammation, and fibrosis markers. The company also has tested an intranasal mode of delivery in mice.
Did someone call blood cleaners?
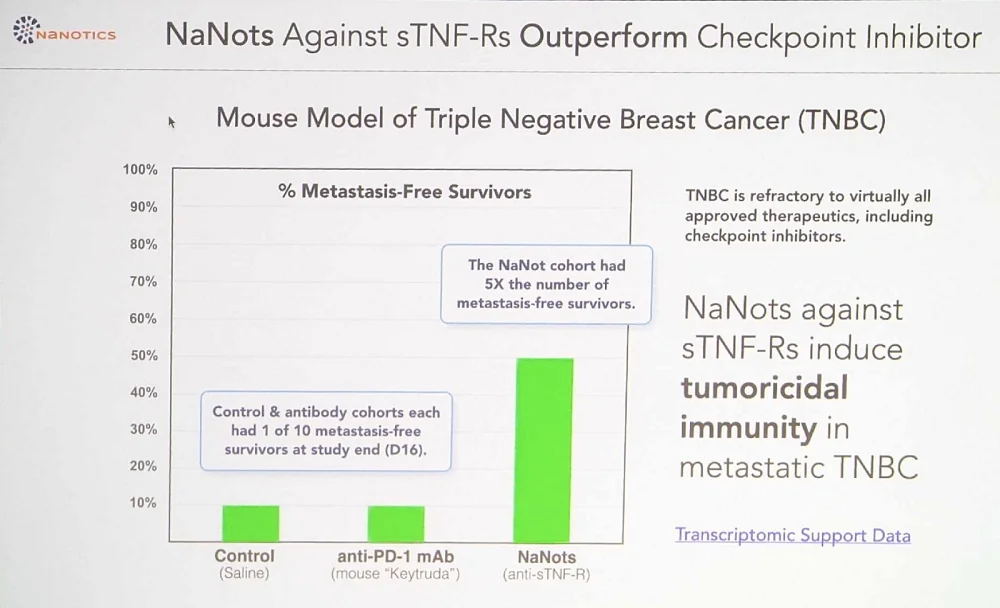
Lou Hawthorne of NaNotics reported on the progress that the company made since his last presentation in Berlin in 2022. NaNotics is working on a universal nanoparticle platform that can clear harmful molecules from the bloodstream. Its most important difference from free-floating antibodies is that NaNots, the company’s proprietary nanoparticles, bind the soluble form of receptors without affecting the membrane form. According to Lou, “soluble factors are often direct mediators of disease and thus more relevant targets.” Many targets have both the essential membrane form and the pathogenic soluble form, and current antibody-based drugs cannot distinguish between them.
Theoretically, NaNots can clear away almost any protein, but currently, the company is after several targets, starting with TNF-α, a cytokine that mediates inflammation and immune response. Immune cells emit soluble TNF molecules. When those molecules bump into transmembrane TNF receptors located on the surface of cancer cells, they transduce an inflammatory signal, essentially a call for reinforcements. Many cancer cells shed soluble TNF receptors to bind this compound in the bloodstream and thus evade discovery. Clearing out those soluble TNF receptors with NaNots should make the tumor visible to the immune system.
Excess amounts of TNF are also behind cytokine storms. TNF-binding nanots lowered mortality in a relevant mouse model. Another TNF-related indication that NaNotics is working on is multiple sclerosis, in which dysfunctional interplay between TNF and TNF receptors causes demyelination in neurons.
NaNots are so versatile that reengineering them for a new target takes just several months. Among the advances since 2022 that Hawthorne mentioned are new encouraging efficacy data in cancer in humanized mice and launching the heavily updated third generation of NaNots.
Dobri Kiprov of Circulate.inc takes a different approach to blood cleansing. His company works on translating experiments in heterochronic parabiosis, in which a young and an old animal’s vasculatures are connected, into actual treatments for humans. Heterochronic parabiosis, pioneered by Irina and Michael Conboy, has been shown to cause some rejuvenation of the old animal.
While we cannot use the same setup in humans, we can perform plasma exchange. However, plasma from a donor can cause compatibility issues, which is why Kiprov’s company basically does plasma dilution by removing the patient’s plasma and replacing it with 5% albumin (the most abundant protein in plasma) in saline solution.
Kiprov reported on a recent TPE (therapeutic plasma exchange) trial that included 40 people. The participants received albumin derived from the plasma of younger people with an average age of 25. The study group showed improvements in metrics of physical strength and SASP. Epigenetic clocks, according to Kiprov, “showed overall positive response to TPE”.
Albumin is known for its anti-inflammatory and antioxidant qualities, so one of the questions being investigated is how much of the positive effect is due to the clearance of harmful “old blood” factors, and how much is due to the introduction of albumin?
Kiprov’s company is already operating a network of clinics providing TPE to private clients.
Unclog ’em!
Two companies had back-to-back presentations on atherosclerosis prevention and reversal. A major cause behind heart attacks and strokes, atherosclerosis is a prolific killer. Incidentally, both Mathew O’Connor of Cyclarity and Reason of Repair Biotechnologies were recently interviewed by Lifespan.io.
Cyclarity focuses on the role of 7-ketocholesterol (7KC) in atherosclerosis. 7KC is an altered form of cholesterol strongly associated with cardiovascular disease – even more so, according to O’Connor, than the infamous LDL cholesterol. 7KC transforms healthy macrophages into diseased foam cells that form the bulk of atherosclerotic plaques.
Cyclarity has been working with a class of molecules called cyclodextrins that can bind to other molecules. Cyclodextrins are widely used and have a favorable safety profile. The trick is to design a cyclodextrin with high affinity and specificity towards 7KC. Cyclarity did it through dimerization, and the resulting product has been shown to rejuvenate foam cells.
Now, Cyclarity is moving into Phase 1 safety trials in Australia. O’Connor hopes that their candidate drug will eventually be able to reduce plaques by 10-15%; a 1% reduction leads to 20% fewer heart attacks.
Repair Biotechnologies has chosen a different approach: removing local excess cholesterol. This is trickier than it might seem, since drugs that can bind to the toxic free cholesterol also bind to cholesterol molecules in cellular membranes, which are indispensable for life. Theoretically, excess cholesterol should be cleared away by the dedicated transport system, but like most other systems in our organisms, it becomes progressively dysfunctional with age.
Repair’s solution is to break down free cholesterol using RNA-loaded LNPs (lipid nanoparticles). After LNPs are taken up by a liver cell, the RNA cargo codes for a fusion protein that can bind free cholesterol. The therapy works by clearing excessive free cholesterol in the liver, which produces systemic benefits.
The treatment has been shown to decrease plaque burden by 17% in a mouse model of accelerated atherosclerosis. Repair is aiming at a clinical trial against homozygous familial hypercholesterolemia, a hereditary disease characterized by abnormally high cholesterol levels, in 2026.
Why repair when you can replace?
Jean Hebert of BE Therapeutics is one of a handful of geroscientists working on a problem that can become a huge roadblock on the way to meaningful life extension: brain aging. Even if we can replace every other organ in the body, we cannot replace the brain without losing the patient’s very self. Thankfully, the brain has a lot of plasticity, rerouting signals away from the damaged parts. Heber gave an example of a patient with brain cancer who had lost the language processing center but not the ability to speak or understand language. This means that, theoretically, we can gradually replace brain tissue “brick by brick”, using tiny patches of healthy tissue, without compromising the brain’s function.
Hebert, a lifelong academic, is new to the biotech scene with his startup, which is less than a year old. The company is in the early preclinical stages but is making good progress. Hebert reported that this company was able to create fully structured and vascularized cortex organoids. Vascularization was solved by including vascular endothelial cells in the graft. The prototype human tissue engrafted in mice developed neuronal connections. In particular, visual cortex neurons mature and become robustly responsive to light.
Our cognitive abilities inevitably decline with age, so gradually replacing our brain tissue seems like a good idea even in the absence of brain diseases. However, the technology will probably be first employed against stroke, traumatic brain injury (TBI), and various types of dementia. Despite its young age, BE Therapeutics, according to Hebert, “has unmatched know-how” since it can produce functional brain tissue rather than one cell type transplants which have numerous limitations.
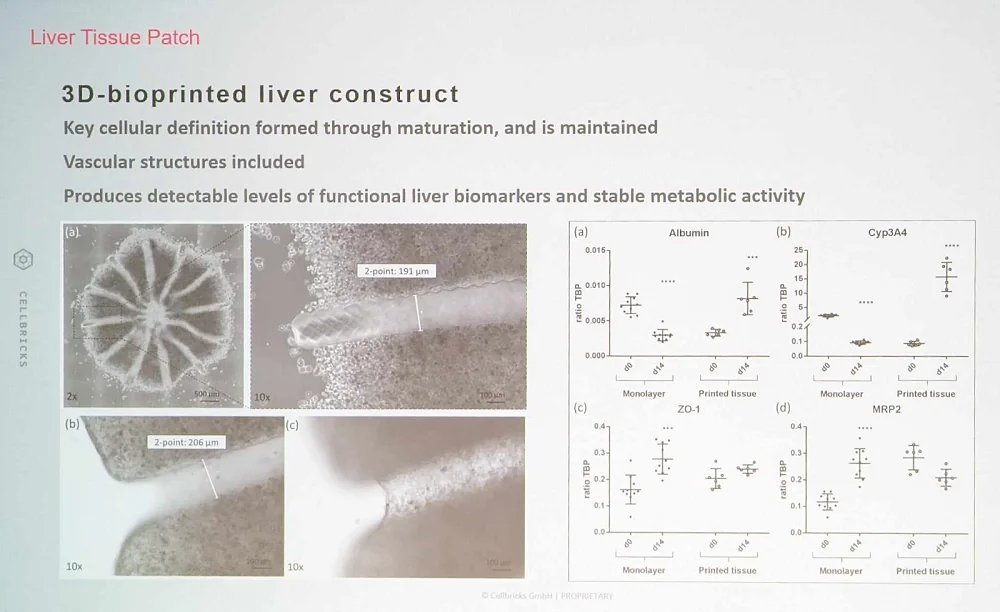
Alexander Leutner of Cellbricks, a young startup based in Berlin, described the company’s attempts to build fully functional blocks of tissue. Cellbricks does it using laser-based multi-material 3D bioprinting, constructing tissue layer by layer, complete with cells of different types, extracellular matrix, and vasculature. The company already has several publications describing bioprinting such tissues as cartilage, liver, and pancreas.
One idea is to build tissue blocks for reconstructive surgery, such as after mastectomy. Another is to implant patches of healthy tissue into a flailing liver, improving its function. In addition to just replacing old or dysfunctional tissue, Cellbricks’ “tissue patches” can also serve as a cell therapy delivery system or as models for diseased tissue such as cancer.
Many other approaches
Alexander Schueller’s company CellVie is among the few in the field that work on mitochondria transplantation. Mitochondrial dysfunction probably contributes to many processes of aging, but, like many other companies, CellVie is focusing on an existing therapeutic indication: ischemia-reperfusion injury (IRI). IRI occurs when blood supply returns to the tissue after an extended lack of oxygen (ischemia). In heart attacks, IRI can lead to further myocardial damage and impaired cardiac function. In strokes, it can exacerbate brain damage, leading to neuronal death, inflammation, and blood-brain barrier disruption.
However, CellVie’s chosen indication is kidney transplantation. When a donor kidney is connected to the patients’ vascular system, IRI happens, damaging the organ and worsening the chances of success. Since mitochondrial damage is a hallmark of IRI, the idea is that injecting healthy mitochondria might help blunt the impact. CellVie has data that shows kidney damage reduced by more than 50% with mitochondria treatment in a pig model of acute kidney ischemia.
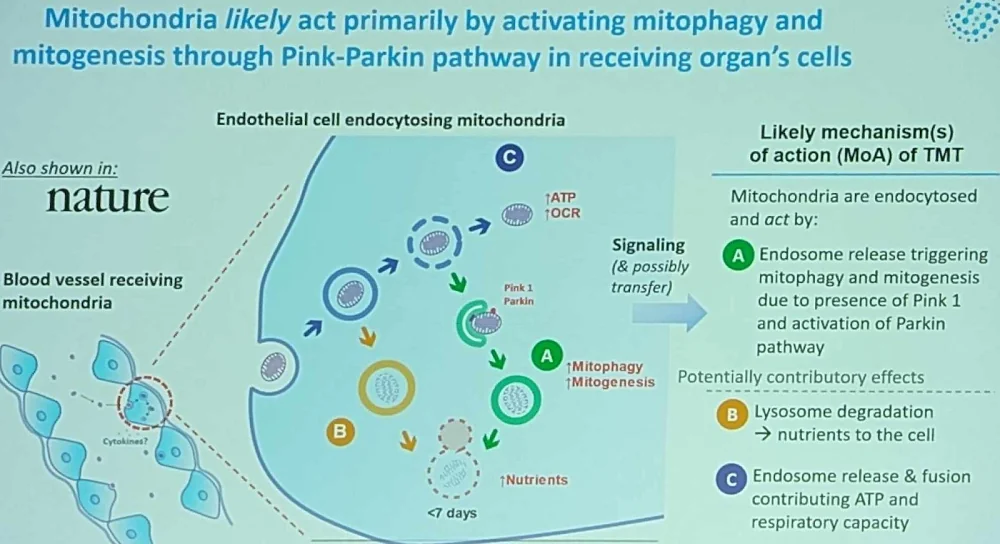
However, one of the hurdles, including in raising money, is that the mechanisms behind the benefits of mitochondrial transplantation are now well known. Schueller thinks his company might be close to answering that question: their research suggests that after arriving at the target cell via endocytosis, new mitochondria activate mitophagy and mitogenesis, rejuvenating the native mitochondrial population.
In other good news, CellVie is collaborating with Boston Children’s Hospital on using mitochondrial transplantation to help myocardial infarction in children. 15 children have been treated so far, with encouraging results.
Greg Fahy of Intervene Immune works on a very specific and important aspect of aging: thymic involution. Thymus is the small organ where T cells mature (hence the “T”). Despite being crucial to our immune system, thymus undergoes involution starting in adolescence, probably because producing new T cells is energetically costly and nature doesn’t really care what happens to us past our reproductive age. By the age of 50, virtually all thymic tissue gets replaced with fat, which leads to impaired immune function, likely contributing to immune-related diseases in older age.
Fahy gained prominence several years ago with his groundbreaking TRIIM trial, in which a combination of human growth hormone (HGH) and metformin was used to try and reverse thymic involution. The treatment led, among other things, to a significant decrease in epigenetic age, providing proof of concept.
Fahy reported on the results of the follow-up trial, TRIIM-XA. It was much bigger than the original TRIIM, with 26 participants, including 6 women (TRIIM had 9, all of them males), and it had both treatment and growth hormone controls. The participants were older and sicker than those in TRIIM, and this time, in addition to epigenetic clocks, functional health metrics were measured too.
TRIIM-XA showed reversal of biological age according to several clocks, including PhenoAge, GrimAge, and DunedinPACE. The treatments also led to improvements in inflammation/immunological profiles, strength, aerobic fitness, blood pressure, and body fat percentage.
Fahy touted a “completely novel agent” that improves epigenetic age reversal and is compatible with the existing TRIIM protocol. Despite Intervene Immune being a relatively new company, their leading candidate for thymus regeneration has been patented and is undergoing Phase 2 trials.
The talk by Lifespan.io Executive Director and Board Member Stephanie Dainow was highly anticipated in light of the recent announcement of a merger between Lifespan Extension Advocacy Foundation (Lifespan.io) and SENS Research Foundation (SRF), two of the longest-running and most prominent non-profits in the longevity field. Dainow presented the two organizations and outlined many synergies, highlighting the underlying shared mission of advancing humanity’s ability to reduce suffering and live longer, healthier lives.
SRF is a research organization with lab facilities in the Silicon Valley with various education programs and damage repair research initiatives. SRF boasts academic and scientific research partnerships as well as startup spinouts and investments. In addition to her role at Lifespan.io, Dainow has assumed the position of Chief Business Officer at SRF and will co-lead the new organization once the merger is completed.
Lifespan.io has become extremely well known for its advocacy efforts to popularize longevity research and accelerate industry progress through strategic media initiatives. While our organization has a respectable record of crowdfunding research, in recent years, Lifespan.io has also become a centralized information and education hub, publishing industry news, creating educational content for our popular YouTube channels, including Life Noggin, and connecting startups with investors through the Longevity Investor Network service platform.
Accordingly, the merger will create a more well-rounded organization, optimally positioned to assume a leading role in the field. Dainow quoted a prominent longevity VC, who had characterized the value the two non-profits bring to the field as “absolutely massive,” adding that these non-profits “are the ones who put the field on the map.” She then encouraged the audience to stay tuned for “many exciting announcements in the coming months on how we plan to evolve, refine efforts and programming, and continue supporting the longevity industry with resources and information that enable accelerated progress.”
The rest of the talk was dedicated to a core advocacy strategy that is often overlooked by the industry and is something that many people at the conference agreed is absolutely essential for improved fundraising and political support. Drawing from Lifespan.io’s decade-long expertise, Dainow shared practical advice on how to communicate longevity to investors who are unfamiliar with the field and outlined the risks of continued poor comms.
There is a general consensus that the main bottleneck for the field is lack of funding. Dainow challenged this assumption and argued that there is plenty of capital. She argued that the actual bottleneck is communication: a skill set that is too often de-prioritized by longevity scientists, researchers, and academic professionals. Dainow shared a recent experience with a group of high-net-worth individuals who had been given a lecture by a prominent scientist about new longevity biotech, only to become frustrated with their inability to understand its content. She emphasized the importance of “meeting people where they are,” that is, of identifying people’s level of scientific comprehension, understanding their mindset and beliefs about aging and never-before-seen-futures, and tailoring the narrative accordingly.

Janine Sengstack of Junevity is another academic-turned-entrepreneur. Her company stems from her PhD work on transcription factors: genes that regulate the expression of several other genes. Junevity analyzes transcription factors’ expression patterns in aging cells to push those cells back towards youthful phenotypes.
Junevity’s goal is to influence factors that are key nodes in the vast network of gene expression. The company uses short interfering RNA (siRNA) to downregulate the expression of the factors that get upregulated with age. This is different from “conventional” cellular reprogramming, in which certain factors are upregulated to push the cell towards de-differentiation. According to Sengstack, downregulation is safer because upregulated transcription factors can bind off-target. Importantly, unlike the full Yamanaka factor cocktail, Junevity’s candidates do not promote cancer-encouraging oncogenes.
The company is developing candidates against obesity and metabolic dysfunction in collaboration with NovoNordisk. A preclinical study showed improvements in liver metabolism in obese mice, with reductions in insulin and creatinine levels, collagen production, and inflammation, after targeting just one transcription factor. Mice on a high-fat diet did not gain weight due to higher metabolism. Junevity’s skin aesthetics program showed improved collagen production and restoration of youthful gene expression across thousands of genes – yet again, following the downregulation of a single transcription factor.
Nicolina Lauc, GlycanAge CEO, told the audience about glycans, complex carbohydrates that play many important roles, mostly by attaching to proteins and lipids and changing their function. Glycans are involved in cell recognition and communication, immune response, protein folding, and various development processes. They even define your blood group.
Glycans are also important in the context of aging: among more than 6,000 molecular traits, 9 of the 20 strongest associations with aging were observed for glycans. This link between glycans and aging enabled GlycanAge to develop its proprietary biological age clock which is available commercially as a blood test. Interestingly, the famous Horvath methylation clock and GlycanAge clock were published on exactly the same date: December 10, 2013.
However, accelerations in glycan and epigenetic clocks do not correlate, meaning thaat they reflect different aspects of aging. This makes glycans a biomarker of aging worth exploring in depth. Notably, the GlycanAge clock does not detect aging deceleration from metformin in healthy people, but it does so in diabetics, which is in line with some recent studies.
Lauc touted her company’s clock’s various advantages over other biomarkers of aging. According to her, it shows little variability between measurements, but is also sensitive to biological changes. Interestingly, while regular optimal physical activity has a very positive effect on the clock, professional athletes fare much worse, on par with people who are inactive and overweight, probably due to increased inflammation. These results support the idea that there is such thing as too much exercise.
Chris Bradley’s company MatterBio aims high: at repairing mutations. Somatic mutations accumulate in every cell in the body. While most of them are neither harmful nor beneficial, the burden always keeps growing. According to Bradley, a typical cell becomes dysfunctional after 1000-5000 mutations. Of course, mutations are also what cause cancer. Another hint at the connection between mutations and aging is the tight cross-species correlation between mutation rate and lifespan.
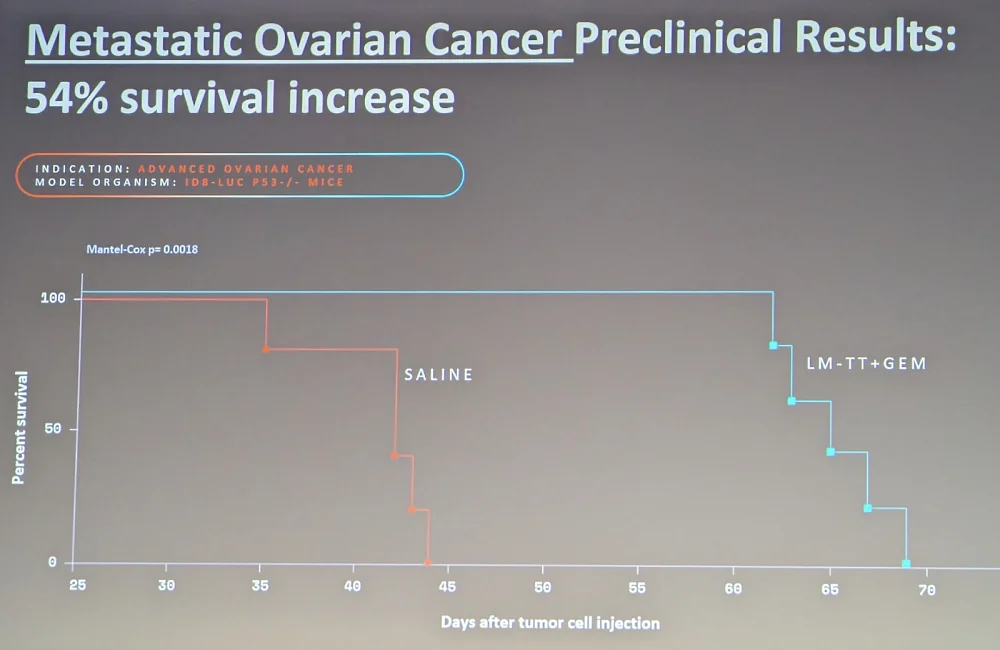
Novel tools give us the ability to identify and fix mutations – which is exactly what MatterBio is trying to do. The three stages of the process are Read, Reverse, and (if you can’t reverse) Remove. The company’s proprietary read tool that can identify single mutations is already available commercially. Another proprietary tool, for reversing mutations, is at a much earlier stage, while their bacteria-based tech for aberrant cell removal is somewhere in the middle, heading into clinical trials for metastatic pancreatic cancer. In pre-clinical studies, it caused a 40% increase in survival for pancreatic cancer and a 54% increase for ovarian cancer. A second treatment cycle doubled survival time in ovarian cancer.