Boosting Autophagy in Astrocytes Might Help Cure Alzheimer’s
- Some cells naturally remove amyloid beta.
With Alzheimer’s disease, most of the focus has been on neurons. A new study’s researchers suggest boosting the process of cellular junk removal in astrocytes, brain cells that perform maintenance tasks, as a new pathway [1].
Looking at a different cell type
Accumulation of amyloid beta, a misfolded protein, is undoubtedly one of the hallmarks of Alzheimer’s disease [2]. However, attempts to cure Alzheimer’s by removing amyloid beta have been largely unsuccessful, although recently, the first moderately effective drugs based on this approach have been approved by the FDA.
It may be possible to instead use the body’s own mechanisms for amyloid beta clearance. Amyloid beta accumulation affects neurons, but it’s the brain’s other cells, collectively known as glia, that are the maintenance workers whose task is to ensure neurons’ unimpeded function. In this new study, a group of Korean scientists investigated the possible role of astrocytes, a type of glia, in amyloid beta clearance, and they arrived at intriguing results.
Fighting the junk
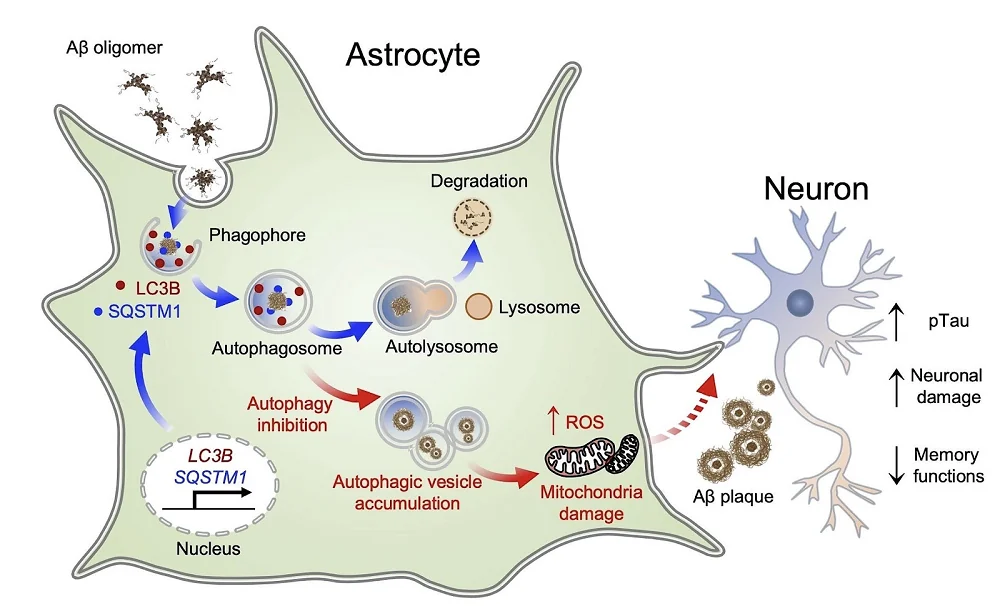
The same group of researchers, as well as others, have already shown that astrocytes take up and degrade amyloid beta [3], but the exact mechanism was not known. This time, scientists focused on the process of autophagy, which removes various cellular junk, such as damaged organelles and misfolded proteins.
Experimenting with mouse astrocytes in vitro, the researchers showed that the introduction of amyloid beta oligomers into this cellular culture causes astrocyte activation and upregulation of genes central to autophagy and an increase in the number of autophagosomes, the intracellular vesicles that participate in autophagy. Interestingly, in neurons, amyloid beta caused a decrease in autophagy levels.
Next, the scientists analyzed tissues from human Alzheimer’s patients. The researchers discovered that the same genes were upregulated in the brains of people with severe Alzheimer’s, but not with mild cognitive impairment (MCI). They also had more activated astrocytes. This suggests that the presence of amyloid beta makes astrocytes work harder in an attempt to remove it.
Returning to cellular cultures, the researchers tried several autophagy inhibitors on mouse and human astrocytes. They discovered that those inhibitors greatly reduced the viability of cells that were challenged with amyloid beta. The researchers saw various signs of mitochondrial dysfunction, suggesting that when autophagy is impaired, this overwhelms mitochondria and leads to increased production of reactive oxygen species.
When the researchers knocked down components of the autophagy process in a mouse model of Alzheimer’s, the mice’s brains showed increased signs of amyloid beta plaque formation. “This result”, they write, “indicates that astrocytic autophagy activation has a critical function in clearing up amyloid beta in AD.” This was accompanied by a decrease in neuronal function and exacerbated cognitive decline.
Boosting autophagy improves amyloid beta clearance
What goes down can come up. When the researchers tried overexpressing the autophagy gene LC3B specifically in astrocytes in Alzheimer’s-prone mice, this significantly decreased the number of amyloid beta plaques in the hippocampus. Boosting autophagy also led to a substantial increase in the number of functional neurons and to an improvement in cognitive and spatial memory functions.
Finally, the researchers transfected human astrocytes with viral LC3B plasmids and, after 24 hours, exposed them to amyloid beta. Just like in mice, LC3B overexpression improved mitochondrial function and decreased the levels of active caspase-3, a marker of cell death.
According to Korea’s National Research Council of Science and Technology, Dr. Ryu and Dr. Suhyun Kim (the first author on the study) said about their research, “Our findings show that astrocytic autophagy restores neuronal damage and cognitive functions in the dementia brain. We hope this study will advance our understanding of cellular mechanisms related to autophagy and contribute to future research on waste removal by astrocytes and health maintenance of the brain.”
Taken together, our studies identify the underlying molecular mechanisms of astrocytic autophagy plasticity and provide critical insights for enhancing the beneficial effects of reactive astrocytes while minimizing the detrimental effects by reactive astrocytes to ameliorate the pathology of AD. In line with this new concept, the pharmacological and genetic modulation of astrocytic autophagy pathway can be considered for boosting the detoxifying machinery of astrocytes under AD stresses and may be a therapeutic strategy for ameliorating AD pathology.
Literature
[1] Kim, S., Chun, H., Kim, Y., Kim, Y., Park, U., Chu, J., … & Ryu, H. (2024). Astrocytic autophagy plasticity modulates Aβ clearance and cognitive function in Alzheimer’s disease. Molecular Neurodegeneration, 19(1), 55.
[2] Rajasekhar, K., Chakrabarti, M., & Govindaraju, T. (2015). Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chemical communications, 51(70), 13434-13450.
[3] Ju, Y. H., Bhalla, M., Hyeon, S. J., Oh, J. E., Yoo, S., Chae, U., … & Lee, C. J. (2022). Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell metabolism, 34(8), 1104-1120.








