Dr. Matt Kaeberlein on WormBot and the Dog Aging Project
- Two very different projects share the same ultimate goal.

Dr. Matt Kaeberlein is a heavy hitter in the longevity field. While his name has become associated with the Dog Aging Project that he started a few years ago, his research interests span much wider. Besides an update on the Dog Aging Project, which is still recruiting dogs, we wanted to talk with Dr. Kaeberlein about his other brainchild, WormBot, a new project that enables scientists to perform mind-boggling numbers of simultaneous experiments in C. elegans nematodes. WormBot has already delivered its first results, and they are exciting.
Let’s start with WormBot.
Conceptually, this project stems from my observation that the field of geroscience has become narrower than it used to be. In part, that’s driven by the fact that we’ve learned enough to be able to formalize some mechanisms of aging, as demonstrated by the hallmarks of aging. We now have things that we can put names on where we sort of understand the mechanisms, but an unanticipated consequence of that is that it narrowed the thinking in the field.
When I first came to the field, people were doing unbiased genetic screens, trying to understand what are the genes that affect lifespan and healthspan in order to get to the mechanisms. We learned a lot from that, obviously, but we don’t do it anymore, in part because of this misconception that we understand aging. It’s probably a natural consequence of formalizing the mechanisms of aging. We’ve created this structure, and it becomes psychologically hard to look outside it.
At any conference on aging, I very much doubt you can find any talks that aren’t directly tied to the hallmarks. One explanation for that could be that we understand it all, but I don’t think that’s true. I think there’s a lot we don’t understand about aging. Then, the question becomes, if you believe there’s a lot more out there that we don’t know, how do you deal with it? The approach that I gravitate towards is to go back to unbiased studies, to look for the things that affect the biology of aging without preconceived notions.
We might think of it as a side effect of specialization.
Absolutely. The more you know about something, the easier it is to continue studying what you know. That works; we’ve learned a lot. Don’t get me wrong: I’m not criticizing the hallmarks of aging. They have been very important and powerful, and we’ve made a lot of progress in the field, but the fundamental question is, do we know everything? Obviously, we don’t. So, how do we look for new things?
If you’re trying to do that for aging, you’re almost stuck using invertebrate models, because you can’t do thousands or millions of experiments in an animal model like a mouse, certainly not in people. I don’t think you can do it using AI only, because the training sets will be constrained to what you already know. So, which invertebrate models make more sense? We’ve done some work in fruit flies, and they’re hard to work with. You can do this in yeast, but we ultimately decided C. elegans was a good starting point.
We wanted to use lifespan and healthspan as endpoints, because they are most informative for the biology of aging. So, is there a way to do a million lifespan and healthspan experiments? We knew about the flatbed scanner approaches that other people have developed in worms. Those are smart approaches and conceptually similar to ours: a flatbed scanner takes timelapse images of worms on plates. Conceptually, that makes a lot of sense, but technology-wise, it’s not very robust. First, flatbed scanners are obsolete; nobody’s developing new flatbed scanner technologies. Second, the software is proprietary, so it’s difficult to change it, to adapt it to your needs.
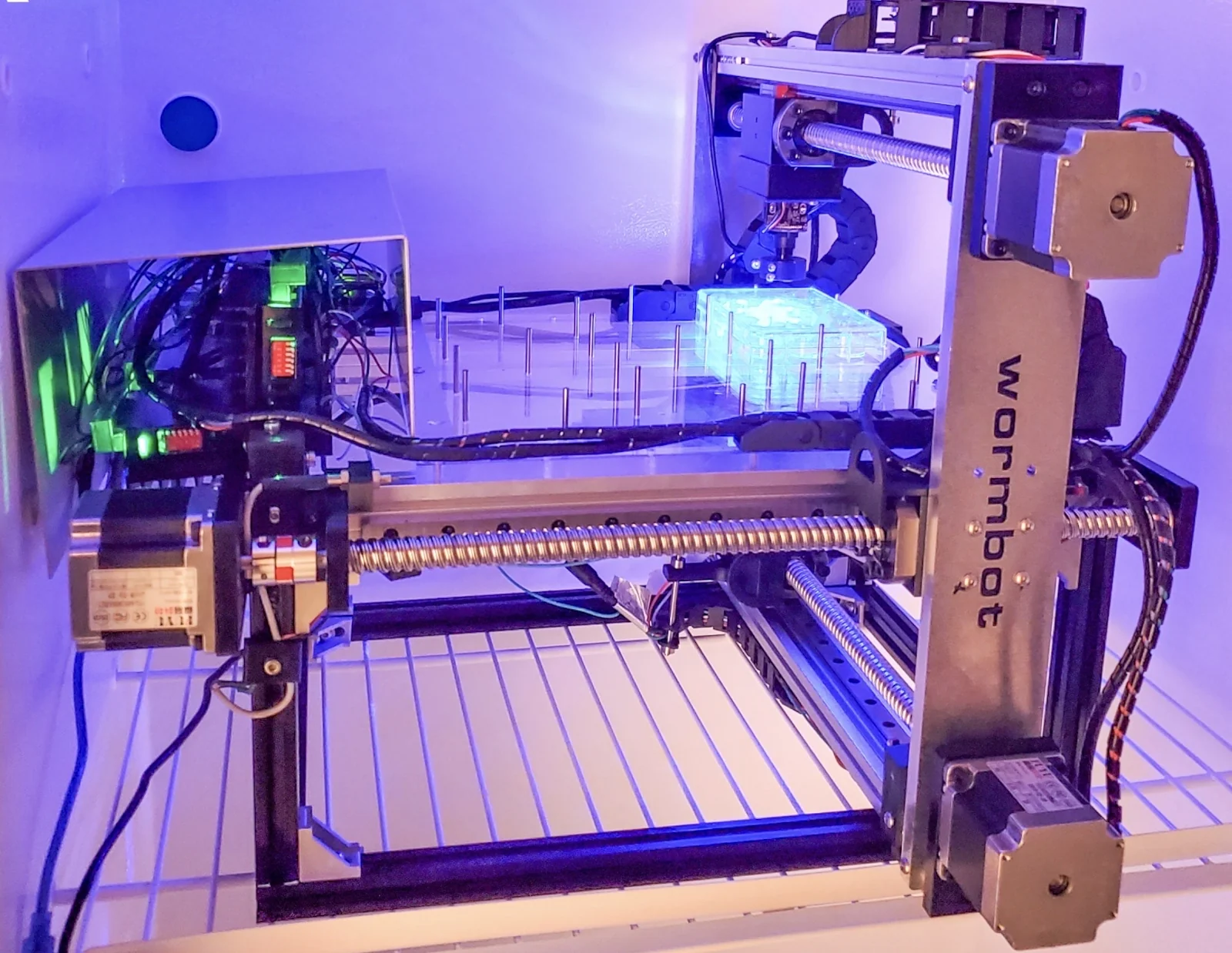
We thought, “What would be another solution that would take this approach and bring it to the 21st century?” The answer was relatively simple: webcams. Instead of putting the plates on a flatbed scanner and running the scanner beneath them, the robot moves the camera, takes a picture, or we can take a movie if we want to, every ten minutes. The other advance that we made is using neural networks to help with identification of the animals and then software that would tell us about movement activity and, ultimately, death over the entire experiment.
The way that the robot physically works, it’s a device that, in principle, you can put as many wells as you want into. We put 144 wells, 12 times 12, 30 animals per well. The robot takes a picture of each well, reaches the end and then starts over, and we compile those timelapse image series for each well. Then we use the neural network and the software to track each individual animal over its lifespan. We’ve already figured out how to tell that the worm has died, but we’re still working on the movement tracking part. It already works well enough to do lifespan experiments.
So, with 144 wells and 30 animals per well, we’re at around 4000 individual animal lifespans per experiment. A worm’s lifespan is a month, so you can do the math to figure out how many devices you need. The nice thing about this technology is that it’s almost infinitely scalable.
Which invites the question, is there a place for citizen science here? I’m talking about schools, maybe individuals.
There certainly could be. One way to do this would be to distribute the robots to thousands of different schools around the world so that they could do their own experiments and collect data. That’s doable, but the challenge would be upkeep and maintenance of the machines. That’s a solvable problem but not trivial.
The other idea we toyed with is that we build a lot of devices, and people would be able to send in their ideas and even their compounds that they want us to test for their lifespan extension potential. It’s kind of a crazy idea, but we thought it was worth the shot. So, you have a centralized facility, but people from anywhere can suggest things to be tested.
Have you thought of offering people a chance to bet on particular interventions or animals?
That’s an interesting idea. We might have to move the factory offshore if we are to bring in gambling. But yes, we could have them bet on worms, right? Which worm is going to live longer? That’s an interesting funding model.
Anyway, you have to start somewhere, and you chose to start from a library of FDA-approved drugs, right?
We have, I think, five devices up and running in the lab right now. Our goal with the company, Ora Biomedical, is to have within 18 months the capacity to test a hundred thousand interventions per year, but we already have a certain capacity to be able to start asking questions.
The concept I suggested was, if we take the unbiased approach, maybe we could find surprising things with large effect sizes. That’s the other thing that has me concerned about the longevity field in general: that the most effective non-genetic intervention we know about is caloric restriction. We’ve known about that since the 1930s. So, why in almost a hundred years haven’t we found anything better than caloric restriction? That, to me, is disappointing.
I guess that’s because we keep bumping into the same pathways.
I think that’s a big part of the answer. Almost everything we know that affects lifespan acts within the same network. Is that because that’s all of aging or because that’s what we’ve been focusing our attention on? Anyway, I’m disappointed that we haven’t found interventions with an even larger effect size and that rapamycin is the most effective intervention we’ve got for increasing lifespan in mice other than caloric restriction.
By the way, what do you think about the latest results on rapamycin and acarbose?
Yes, it looks like there’s some additive effect in combining them. I think it’s great. The disappointing part of it was that the controls weren’t built into the same experiment. That’s a criticism that’s always going to be raised, much like with the rapamycin and metformin experiment, that you didn’t have rapamycin alone in the same concentration. Even still, I think it’s encouraging from the perspective of combinatorial interactions, which is also where we looked first.
There are a few interesting questions. One, if you had tested a million compounds, would you find something better than rapamycin? I would be shocked if you wouldn’t, but that’s an unknown at this point. Another question is, “What happens when we start combining more than one thing that affects lifespan?”
There’s reason to believe that at least in some cases, combining two or three things could be beneficial, and we wanted to be able to explore that. This is where we decided to start with WormBot, because we felt like we had enough capacity to test individual drugs, and, in select cases, combinations.
The strategy we settled on was to start from FDA-approved drugs because not only are they approved for human use, but, usually, something is known about their mechanisms of action. They are also usually quite potent, so the chance to get a sizeable effect is higher.
Second, we said we can’t do an n-by-n matrix of all possible interactions, but we can take one FDA-approved drug that we know to affect aging and then combine it with individual FDA-approved drugs that we know nothing about aging-wise. So, we settled on metformin, because in worms, metformin is very consistent, it gives them about 15% increase in lifespan.
So, it’s not the largest increase, but it’s consistent?
That’s right. We designed a very simple experiment: take several drugs and test each drug by itself and in combination with metformin. In every run, there was vehicle control, drug alone, metformin alone, and their combination. Another change that we made is that instead of starting with wild-type worms, we started with the strain called GMC-101, which is very short-lived, because it expresses amyloid beta in body wall muscles. We did that to be able to screen through the library faster. It’s a legitimate criticism to question how much those worms can tell us about aging, but I think it’s a pretty good predictor of lifespan in wild-type worms. This is something we are testing now.
What we found was that metformin was rock-solid, it would give a 10-20% increase in lifespan in 95% of the experiments, but we had some completely unexpected interactions. We identified new FDA-approved drugs that are way more effective than metformin: 50-70% effect. Most interestingly, we identified synergistic interactions that extend lifespan by 300%: huge effect sizes. At least in some cases, we know the biochemical mechanism of action of the second drug, and it makes sense to us.
First, some specific interactions are super-interesting, and it obviously is going to be something that the company pursues going forward. We’ll publish all of this as well, but what I think is fundamentally more interesting is the type and frequency of the interactions that we see between metformin and these other drugs.
We have, I think, three cases now where we get these big synergistic interactions. I’m very encouraged by the fact that we are seeing effects that are larger than anything we’ve seen from single drugs or genetic alterations. It’s telling me that this approach has merit and that we have to scale those experiments up. I’m confident we’re going to find some interesting stuff.
Where does it stand now? I understand that you tried to get funding from the NIA and decided to take the commercial route instead.
It’s very hard, nearly impossible, to get NIH funding for a tool that does unbiased screening. As soon as you start talking about anything that even smells like unbiased screening, the reviewer says, “that’s a fishing expedition, you don’t have a mechanistic hypothesis.” This will kill your grant right away.
But someone has to do it. This is frustrating.
I think it’s a culture that’s evolved there. This isn’t unique to NIA, but it’s something that happens when the field becomes more mechanistic and specialized. Yes, mechanistic studies are critically important, and we do such studies in my lab, but the culture evolves where if it doesn’t fit into that framework, you can’t fund it anymore.
So, I could continue banging my head against the wall writing grant after grant, but we decided that there’s an opportunity to spin this out as a company. It’s not an easy sell as a company because it doesn’t fit into the culture of traditional biotech either.
Right, because what’s the product?
That’s a good question. I am certain that we can come up with interventions, combinations with FDA-approved drugs or new drugs, that are at least twice or even an order of magnitude more effective than the interventions we have today that target the biology of aging. I think that’s a pretty goddamn good product.
I certainly will. But there’s a major problem with repurposing drugs, isn’t it? Little commercial incentive here.
I’d say this is an open question. There’s certainly a perception that there’s no path to profitability with repurposing drugs. I’m not sure it’s true. It’s an unusual path, but at least in some cases, you can get some kind of unique IP around it. Anyway, you don’t have to start with FDA-approved drugs. Or you start with them and then, if you know what the target is, in theory, you could develop or identify new drugs that work on the same target.
For example, I mentioned this one drug that interacts with metformin. We know what the biochemistry is, what the target is, and we have this synergistic effect. Our prediction is that other drugs that hit that biochemical target will show the same synergy with metformin. If that’s true, there are other drugs out there that have IP around them.
Yes, the company has absolutely gotten that question, “what’s the product,” and that’s a challenge. You have to convince the investor that the idea is solid enough and that it’s important, that identifying interventions that are significantly better than what we have today is important. We’ll see how successful we are.
Maybe the platform itself can be the product.
It could be, the company could sell bots, but that’s not the path we’re taking for various reasons. I think the data itself could be a product. Imagine a database with one hundred thousand longevity experiments. Is there value in a proprietary database of tested compounds and combinations? I think so.
The one thing that gives me optimism is that there are funders in the longevity space in particular who are mission-oriented and who recognize what some of the outstanding questions in the field are. The company’s been successful in getting initial funding, and the seed funding round will hopefully be closed early next year. Then it’s off to the races.
Let’s switch gears and talk about the Dog Aging Project. Last time we talked, early last year, it was about to begin.
Well, it’s begun. To refresh, there are two parts to this project. There’s the longitudinal study of aging, which is not interventional. We now have survey data on more than 40,000 dogs. We’ve started publishing papers, including in Nature earlier this year. The first data release is now publicly available. We are analyzing data and making it available to the scientific community, which feels really good.

DAP founders Dr. Matt Kaeberlein, Dr. Kate Creevy, and Dr. Daniel Promislow with University of Washington mascot Dubs at the 2022 Dog Aging Project Annual Meeting
Do you have any insights to share that might be valuable for dog owners?
It’s very early, but we have some interesting stuff. First, we did a study on feeding frequency. The idea behind it came to me when I was writing a review on caloric restriction and anti-aging diets that was published in Science at the end of 2021. To reiterate, caloric restriction is the most robust and effective way to increase lifespan, at least in rodents. It doesn’t always work – some genetic backgrounds are harmed by caloric restriction – but across the board, it’s the most effective intervention we’ve got.
There are many variants of caloric restriction that became popular, like intermittent fasting, time-restricted feeding, and ketogenic diet. It wasn’t clear to me that all those things in the absence of caloric restriction per se provide any benefit at all, at least for lifespan. My intuition is that intermittent fasting has a tiny effect, maybe 7-8%, but it’s really the caloric restriction that gives you most of the benefits. So, it occurred to me that dogs provide a great opportunity to study this.
I think any dog owner would agree.
Absolutely. Some feed their dogs three times a day, some twice a day, some once a day. So, we’ve got this population where different dogs are fed different numbers of times each day, and we can ask a very simple question: is there any correlation with age-related diseases?
The statisticians delved into the data and came up with this simple design, where we bin dogs into two groups – those fed once a day and all the others – and look across nine different diseases and cognitive function. I didn’t think this was going to work, because I didn’t really believe in time-restricted feeding.
But, in every case, the directionality was clear: dogs fed once a day were less likely to have been diagnosed with a particular disorder. In six of the nine cases, this was statistically significant, and also for cognitive function. I think it’s interesting, although it doesn’t prove causality, and at least one of the possibilities is that dogs fed once a day are just less likely to be obese, they have better metabolic health.
Did you control for this?
We will be able to do this in the future, when we have more information about diet. We don’t know a lot about the metabolic health of those dogs, but at least we’ll be able to get a better look at obesity. It allows us to develop certain testable hypotheses. I’m interested both in obesity and in diet composition.
Another interesting thing was to look for correlations between cognitive function and environmental metrics. Not shockingly, exercise has a huge role in the risk of developing dementias. Dogs that are regularly exercised have up to sevenfold risk reduction. It’s also reassuring to see that what we know about humans in this respect also holds up for dogs. This allows us to go back and do more quantitative studies. We only had crude owner-reported data, but now we’d like to put activity monitors on some dogs and do more precise measurements of cognitive function.
Is there any dog equivalent of exercising the brain?
I would speculate that a variety of different types of play would probably fall into that category, or maybe dogs that are true working dogs, but I don’t know if anybody has ever formalized that, and I would be very surprised if anybody has ever looked at the impact of that as an intervention. If you spend 20 minutes a day playing a game with your dog that forces them to use different aspects of cognition, would that have a protective effect on dementia? It’s a super interesting question
One of the people on our team, Evan MacLean, is a neuroscientist, and he’s developed a series of games owners can play with their dogs that measure different aspects of cognitive function, so we’re incorporating those tests into some of our more specialized studies, including the clinical trial of rapamycin. So, it would be interesting to think about it.
Where does TRIAD, the rapamycin arm of the Dog Aging Project, currently stand?
That trial is under way. Several of our first dogs have already completed their first six-month appointments, and a few are nearing their one year appointments. Just to remind you, this is a double-blind, placebo-controlled, randomized clinical trial, the goal of which is to determine whether rapamycin can impact lifespan and healthspan in companion dogs. The primary endpoint of the trial is lifespan, and then secondary endpoints include a bunch of metrics such as cardiac function, neurological function, cognitive function, activity, disease incidence.
Initially, the size was 350 dogs, and then we got a donation to expand it to 580. This is enough to give the study the power to detect a 9% change in lifespan. The dogs have to be at least seven years old to participate in the study, because we want a middle-aged population, and 40 to 110 pounds because larger dogs age faster. They also have to be of normal health status without preexisting diseases.
The dogs come to our sites. Right now, we have nine sites, all in veterinary teaching hospitals, but we are close to finalizing an expansion to 20-30 sites, hopefully, in the next three months.
Are you still recruiting? We’d love to get the word out.
Absolutely. The problem has been that we only have those nine sites, and many veterinary teaching hospitals, for historical reasons, are located in rural areas. Our expansion will also include some private clinics in urban centers. We hope this will help with better recruitment. We have about a hundred dogs in the study right now, so we still have a long way to go to meet our recruitment goals.
My realistic estimate is that we’ll complete recruitment by the end of 2023. The design is a one-year treatment, two-year follow-up. Hopefully we’ll have all dogs through the treatment phase by end of 2024, and the study will be completed by end of 2026. It’s frustrating to me how long it takes, but if you think about the same study in humans, we’re a couple of decades ahead of it.
Do you have an elevator pitch for dog owners? Why should they participate?
Their dogs are part of their families, so the goal here is to extend healthy longevity for a family member. While we can’t guarantee that your dog will be receiving the drug, and we don’t know if rapamycin is going to work, we have good reasons to believe that it will. We need to do this trial to find out. If it’s successful, the potential impact on the quality and quantity of life of those family members will be immense: maybe a 15% increase in healthy longevity. So, it’s important for the dogs, their owners, and for moving forward with similar studies in humans.
You are a well-known enthusiast of rapamycin. Has your opinion about it changed lately?
I think it’s still a fact that we don’t know what the risk profile of rapamycin use is in people who are of normal health status, because it’s usually used for organ transplantation. That’s a question we simply need to get an answer to, otherwise it’s going to be hard to overcome rapamycin’s bad reputation. Certainly, from my own personal experience and anecdotal information collected from people who are using rapamycin, I am confident that rapamycin has beneficial effects for some people.
You do have an interesting anecdotal experience with rapamycin, right?
In my case, within a few weeks of starting rapamycin, I noticed a significant improvement in a condition called adhesive capsulitis, or frozen shoulder. It’s fairly common in middle-aged people, it’s extremely painful, and there’s little that can be done.
It got to the point where I couldn’t go outside and throw a ball with my son, I couldn’t sleep well at night because of the pain. After doing some research, I realized it was an inflammatory condition. Rapamycin is known to have beneficial effects on age-related sterile inflammation, so I thought I’d give it a try. In two to four weeks, I noticed a significant improvement in range of motion, in pain. In the end of the ten-week cycle, I got maybe 90% of motion range back and very little pain. Sure, it could be a placebo effect, but it was so painful and debilitating, so I would be shocked if it was placebo effect.
But we have good anti-inflammatory drugs, why would rapamycin work better?
We do have good anti-inflammatory drugs, but we don’t have drugs that work precisely the same way that rapamycin does. I’m not an immunologist, so it may be naïve, but it’s my intuition from reading a lot of literature that rapamycin seems to be very effective against age-related sterile inflammation.
It probably has something to do with its action on different subsets of T cells. So, it’s not a general anti-inflammatory drug, it doesn’t work in the same way. Why would it be better for this particular condition? I don’t know, I don’t even know it is, this is my n = 1 story. But I think this has something to do with the mechanisms that underline this sterile inflammation that goes with aging, and it is probably due to the fact that MTOR is a potent regulator of aging biology.
There’s been some interesting research about early-life rapamycin treatment, with “rapamycin memory” probably working via autophagy.
I think this is very early. Those are studies in drosophila and mice. We already knew you can get a persistent effect from a single treatment with rapamycin. What we did in my lab was take 20-month-old mice, treat them for 12 weeks, and do follow-up. We have persistent effects on cancer and also longevity benefits. The new data says that you can have some effect even from a very early life treatment. I don’t know if those are the same mechanisms or not. My intuition is that these are different mechanisms, but I think we just don’t know yet at this point. I probably should say they are overlapping but not identical. But, yes, it’s very intriguing.
I think it’s an interesting basic science question, but I don’t see direct therapeutic use. I don’t think any serious person would consider giving rapamycin to pregnant women or kids. If we can understand the biology there, potentially there is therapeutic application. I think epigenetics is the obvious place to look, it’s the easiest explanation, but we’ll have to do more experiments to find out.
Which directions in geroscience are currently underrated or overrated?
I would say I’m both excited by epigenetic reprogramming and I think it’s currently overrated. This is probably the most exciting intervention we have on the table today that could be better in terms of effect size than rapamycin and caloric restriction. I also think it’s been greatly oversold, given where the data is today. We just need to do more experiments. Show me that you can double lifespan in a mouse, and I’d be really excited.
Anything else you are excited about?
Conceptually, I’m excited about what else is out there; what don’t we know? I hope people will start exploring this idea a bit more. There’s so much cool stuff to be discovered, but we have to start looking for it. Another thing I’m excited about is that we’re actually starting to see serious science-based efforts to take the biology of aging and what we know about it and target it in clinical studies. These are really early days, but we’re getting there, and serious people are starting to do real clinical trials or apply science-based longevity medicine in people, and in dogs! I think the next ten years are going to be very exciting.







