Resveratrol: Benefits, Side Effects, and Research
Resveratrol is a popular supplement especially in the longevity community. There are numerous supplement companies touting it in adverts for weight loss and longevity. But do these promises really stack up?
What is Resveratrol?
Resveratrol is a naturally occurring polyphenol. These are compounds found in plants that act as antioxidants. Antioxidants can help protect the body from damage. This damage can increase the risk of cancer and heart disease.
It has many potential benefits. These include better mitochondrial function, protection against obesity and related diseases, reduced inflammation, and slower cancer cell growth. It also helps with heart disease [1-3].
However, clinical trials have produced mixed results [4]. When taken orally, evidence indicates that its bioavailability is relatively low. This is because it is rapidly metabolized and removed from the body.
Sources of Resveratrol
Resveratrol is a naturally occurring compound and is present in a number of food sources.
- Peanuts
- Pistachios
- Blueberries
- Cranberries
- Soy
- Grapes
- White wine and in higher amounts in red wine
- Cocoa and dark chocolate
Benefits of resveratrol
Caloric restriction has been shown to increase longevity across species [5]. Meanwhile, national overconsumption of calories has worsened the obesity epidemic [6]. This situation has prompted researchers to search for compounds that trigger the caloric restriction response.
Studies on caloric restriction in yeast found that the enzyme SIR2 plays a key role. It helps the body respond to nutrient scarcity [7]. Sirtuins are NAD-dependent deacetylases that are associated with longevity.
After this discovery, many molecules were tested to find activators of SIRT1, the human version of SIR2. Resveratrol was identified as potentially effective [8].
Resveratrol was first characterized as a cyclooxygenase (Cox) inhibitor and a potentially chemoprotective molecule [9]. Cox inhibitors are used to reduce inflammation and pain and treat certain cancers.
Since its discovery as a calorie restriction mimetic studies have shown it to have beneficial effects. For example, in cardiovascular disease [10], metabolic disease [11], cancer [9], and neurodegeneration [12]. In this way, resveratrol may potentially improve health in many ways. This depends on the system and which mechanisms are most active.
Its systemic effects come from several mechanisms. These include direct antioxidant activity and inhibition of cyclooxygenase 2 (Cox-2). It also activates Sirt1 and inhibits F1-ATPase.
Additionally, it blocks estrogen receptors and affects biotransformation enzymes. It inhibits cell growth and induces cell death (apoptosis). It also prevents tumor invasion and angiogenesis, along with having anti-inflammatory effects.
Resveratrol and cardiovascular disease
Resveratrol has been shown to inhibit vascular cell adhesion molecule (VCAM) expression in tissue culture [13].
VCAM is found on the surface of blood vessel cells. It appears when there is oxidative stress. These proteins are expressed on the surface of endothelial cells. They help anchor and allow entry for inflammatory white blood cells.
These cells migrate across the endothelium into the middle layer of the artery, where they can become foam cells. Foam cells make up the bulk of arterial plaque [14]. This suggests that taking resveratrol may potentially slow atherosclerosis. It does this by keeping immune cells from invading the artery [15].
Resveratrol inhibits vascular smooth muscle cell proliferation. Hypertension increases mechanical stress on arterial walls. The body reacts by increasing the growth of vascular smooth muscle cells. These cells line the middle layer of the artery.
This can cause the artery to thicken and become less distensible, leading to chronic hypertension and its consequences. It has been shown to inhibit the proliferation of VSMCs in tissue cultures and in the body [16,17].
Resveratrol upregulates nitric oxide synthase. The endothelium of the artery contains an enzyme that catalyzes the production of nitric oxide (NO). NO induces relaxation of the arterial tree. Dysregulation of NO homeostasis can contribute to increased blood pressure.
In cultured endothelial cells, resveratrol has been shown to stimulate endothelial nitric oxide synthase. This suggests that it may increase nitric oxide synthesis [18].
Resveratrol has been shown to inhibit platelet activation and aggregation. These processes are central to clot formation, which is potentially the most dangerous risk factor in cardiovascular disease [19].
Resveratrol, diabetes, and obesity
More than one-third of United States adults suffer impairments in glucose metabolism. These problems include insulin resistance, issues with insulin secretion, and problems with insulin receptor signaling. Also difficulty using fat for energy, changes in lipid profiles, and increased levels of pro-inflammatory cytokines [20-21].
Resveratrol improves insulin sensitivity, glucose tolerance, and lipid profiles in obese or metabolically abnormal people [22]. This means that it has the potential for helping people to lose weight and control blood glucose. Excess body weight is a key risk factor for a number of age-related diseases.
It can lower fasting glucose and insulin levels and also improves HbA1c. Additionally, it increases HDL and reduces LDL cholesterol and high blood pressure [23]. Resveratrol was found to improve the activity of metabolic sensors, including SIRT1 and AMP-activated protein kinase (AMPK) [3].
Resveratrol and neurodegeneration
Resveratrol has been shown to reduce oxidative stress in nerve tissue and decrease neuroinflammation. It also appears to clear β-amyloid and α-synuclein plaques, and improve mood and performance on cognitive tests.
Age-related deficits in hypothalamic function result in poor memory. Resveratrol supplementation in rats helps create new synaptic connections. They also support blood flow changes in older rats.
In tandem, improvements in memory, learning, and mood also occurred [24]. Studies of human supplementation with resveratrol show similar cognitive improvements [25].
The buildup of β-amyloid plaques is related to Alzheimer’s disease. Similarly, α-synuclein is linked to Parkinson’s disease. This buildup is connected to cognitive decline in both diseases. In mouse studies, resveratrol has been shown to slow or reverse the buildup of these aggregates.
It also helps cells survive and reduces neuroinflammation. Microglia, cells in the brain that perform immune surveillance when activated, were found to be inhibited by resveratrol [28,29].
It has been shown to reduce the mitochondrial dysfunction and oxidative stress, which leads to numerous neurological disorders [30-31].
A study of older overweight adults took resveratrol (200mg) and quercetin (320mg) daily for 26 weeks. This led to significant improvements in memory [25].
Resveratrol and longevity
Resveratrol increases lifespan in yeast by 70% [32]. In worms, fruit flies, and vertebrate fish, it also significantly extends lifespan [33-34]. However, in mice, the situation becomes more nuanced.
Resveratrol has been shown to increase the lifespan of mice on calorie-rich diets. A study showed that their average life expectancy was the same as normally fed mice [35].
At present, there are no studies that link with extended maximal lifespan in humans. There is also no study that measures lifetime consumption of resveratrol [32].
Resveratrol and cancer
Resveratrol is a phytoalexin, a substance produced by certain plant species at sites of pathogen infestation [36]. It functions by inhibiting the growth of bacteria or fungi. Which raises the question of how it might affect eukaryotic cell growth and proliferation.
Resveratrol can slow down the growth of many human cancer cells. This includes cells from the breast, colon, liver, pancreas, prostate, skin, thyroid, white blood cells, and lungs [37-39]. In total, it has been shown to inhibit the initiation, promotion, and progression of cancer.
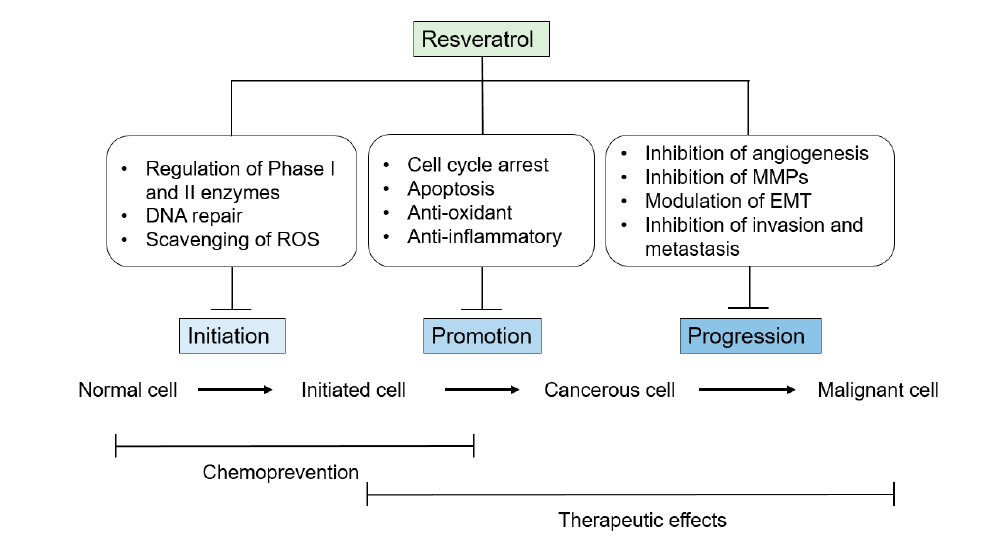
In rodent models, it stops the development of cancer cells in several ways. It directly and indirectly removes reactive oxygen species. It also blocks enzymes that change carcinogens into their active forms. Additionally, it reduces DNA repair processes and anti-apoptotic signaling in cancer cells.
For carcinogens to cause cancer, they must be activated by enzymes in the body, such as cytochrome p450. Resveratrol inhibits cytochrome p450, thus nullifying the activation of carcinogenic compounds [40].
Resveratrol was found to lower the levels of proteins needed for normal DNA repair in cancer cells. One of these proteins is DNA ligase I, which helps reconnect broken parts of DNA [41]. Its antioxidant activity helps remove reactive oxygen species [42]. It also reduces inflammation and promotes cell-cycle arrest and apoptosis [43].
Finally, resveratrol slows cancer growth in several ways. It stops new blood vessel growth [44]. It also blocks matrix metalloproteinases [39,45]. Additionally, it prevents the invasion and spread of cancer in the body [46-47].
The use of resveratrol as a cancer therapeutic in humans has been difficult to assess. This is due to its poor bioavailability. A study looked at how it affects colon cancer. It found taking 4g to 8g daily for a month slightly reduced cell growth [48].
New micronized and liposomal formulations of resveratrol have yielded more useful results. Micronized formulations increase bioavailability by breaking the product into a finer particulate with more surface area. Using a more bioavailable resveratrol, researchers found an increase in the death rate of colorectal cancer cells [49].
Another study tested a resveratrol treatment for the blood cancer multiple myeloma. It found many side effects, including kidney failure. However, kidney failure is very common in multiple myeloma. It is not clear if these complications were due to resveratrol or a natural result of the disease [50].
New formulations that utilize nanocarrier technology promise more targeted delivery with high bioavailability. These formulations include the use of liposomes, polymeric nanoparticles, ethosomes, phytosomes, and nanotubes. Some of these applications are topical, and others are oral. Targeted delivery greatly reduces off-target effects and toxicity while requiring less resveratrol [51].
Side effects of resveratrol
Studies in dogs suggest that resveratrol can be consumed in doses as high as 600 mg/kg in dogs with minimal safety concerns. That translates to approximately 45g/day for a 150-pound individual [52].
Significant side effects in humans appear to be rare. That said, few clinical trials have attempted to determine what the limits for resveratrol consumption are [53].
Some studies have indicated a risk of mild to moderate gastrointestinal side effects. These include nausea, flatulence, and abdominal pain, in people consuming more than 1g a day. A small study found that 5 grams a day resulted in no serious side effects [54].
Pregnancy, lactation, Estrogen, and resveratrol
No studies have established safe upper limits for resveratrol consumption in pregnant and lactating women. While there is a source of resveratrol in red wine, pregnant and lactating women are cautioned against its consumption.
Resveratrol is a compound that acts like estrogen. Studies show it can block the estrogen receptor and also mimic estrogen. Women with a history of estrogen-driven cancers are cautioned against resveratrol consumption. More studies are needed to understand how resveratrol affects them.
Drug interactions
Anticoagulant drugs significantly increase the risk of bruising and bleeding. Resveratrol is known to inhibit platelet aggregation in vitro. Therefore, resveratrol combined with anticoagulant drugs of any kind could potentially result in uncontrolled bleeding [55].
While resveratrol’s inhibition of cytochrome P450 suppresses carcinogen activation, P450 is also used to metabolize many drugs. This suggests that resveratrol intake could increase the potency and risk of toxicity of these drugs [56].
Disclaimer
This article is only a very brief summary. It is not intended as an exhaustive guide and is based on the interpretation of research data. That data is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you.
We do not support the use of supplements or any product vendors. All discussions here are for scientific interest.
Literature
[1] Tabrizi, R., Tamtaji, O. R., Lankarani, K. B., Akbari, M., Dadgostar, E., Dabbaghmanesh, M. H., … & Asemi, Z. (2020). The effects of resveratrol intake on weight loss: a systematic review and meta-analysis of randomized controlled trials. Critical reviews in food science and nutrition, 60(3), 375-390.
[2] Higashida, K., Kim, S. H., Jung, S. R., Asaka, M., Holloszy, J. O., & Han, D. H. (2013). Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS biology, 11(7), e1001603.
[3] Price, N. L., Gomes, A. P., Ling, A. J., Duarte, F. V., Martin-Montalvo, A., North, B. J., … & Sinclair, D. A. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell metabolism, 15(5), 675-690.
[4] Bitterman, J. L., & Chung, J. H. (2015). Metabolic effects of resveratrol: addressing the controversies. Cellular and Molecular Life Sciences, 72, 1473-1488.
[5] Hector, K. L., Lagisz, M., & Nakagawa, S. (2012). The effect of resveratrol on longevity across species: a meta-analysis. Biology letters, 8(5), 790-793.
[6] Sarma, S., Sockalingam, S., & Dash, S. (2021). Obesity as a multisystem disease: Trends in obesity rates and obesity‐related complications. Diabetes, Obesity and Metabolism, 23, 3-16.
[7] Kaeberlein, M., McVey, M., & Guarente, L. (1999). The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes & development, 13(19), 2570-2580.
[8] Chen, D., & Guarente, L. (2007). SIR2: a potential target for calorie restriction mimetics. Trends in molecular medicine, 13(2), 64-71.
[9] Subbaramaiah, K., Chung, W. J., Michaluart, P., Telang, N., Tanabe, T., Inoue, H., … & Dannenberg, A. J. (1998). Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. Journal of Biological Chemistry, 273(34), 21875-21882.
[10] Bonnefont-Rousselot, D. (2016). Resveratrol and cardiovascular diseases. Nutrients, 8(5), 250.
[11] Hou, C. Y., Tain, Y. L., Yu, H. R., & Huang, L. T. (2019). The effects of resveratrol in the treatment of metabolic syndrome. International journal of molecular sciences, 20(3), 535.
[12] Komorowska, J., Wątroba, M., & Szukiewicz, D. (2020). Review of beneficial effects of resveratrol in neurodegenerative diseases such as Alzheimer’s disease. Advances in Medical Sciences, 65(2), 415-423.
[13] Carluccio, M. A., Siculella, L., Ancora, M. A., Massaro, M., Scoditti, E., Storelli, C., … & De Caterina, R. (2003). Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arteriosclerosis, thrombosis, and vascular biology, 23(4), 622-629.
[14] Ferrero, M. E., Bertelli, A. A., Fulgenzi, A., Pellegatta, F., Corsi, M. M., Bonfrate, M., … & Bertelli, A. (1998). Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. The American journal of clinical nutrition, 68(6), 1208-1214.
[15] Stocker, R., & Keaney Jr, J. F. (2004). Role of oxidative modifications in atherosclerosis. Physiological reviews, 84(4), 1381-1478.
[16] Mnjoyan, Z. H., & Fujise, K. (2003). Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: a role of p53–p21WAF1/CIP1 pathway. Biochemical and Biophysical Research Communications, 311(2), 546-552.
[17] Khandelwal, A. R., Hebert, V. Y., & Dugas, T. R. (2010). Essential role of ER-α-dependent NO production in resveratrol-mediated inhibition of restenosis. American Journal of Physiology-Heart and Circulatory Physiology, 299(5), H1451-H1458.
[18] Takahashi, S., & Nakashima, Y. (2012). Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. British journal of nutrition, 107(6), 774-780.
[19] Yang, Y. M., Chen, J. Z., Wang, X. X., Wang, S. J., Hu, H., & Wang, H. Q. (2008). Resveratrol attenuates thromboxane A2 receptor agonist-induced platelet activation by reducing phospholipase C activity. European journal of pharmacology, 583(1), 148-155.
[20] US Department of Health and Human Services, “National Diabetes Statistics Report, 2020,” Natl. Diabetes Stat. Rep., p. 2, 2020.
[21] Zhu, X., Wu, C., Qiu, S., Yuan, X., & Li, L. (2017). Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutrition & metabolism, 14, 1-10.
[22] Szkudelski, T., & Szkudelska, K. (2015). Resveratrol and diabetes: from animal to human studies. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1852(6), 1145-1154.
[23] Brasnyó, P. (2011). National Diabetes Statistics Report, 2020and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr, 106(3), 383-389.
[24] Wang, Z. M., Zhao, D., Nie, Z. L., Zhao, H., Zhou, B., Gao, W., … & Yang, Z. J. (2014). Flavonol intake and stroke risk: A meta-analysis of cohort studies. Nutrition, 30(5), 518-523.
[25] Witte, A. V., Kerti, L., Margulies, D. S., & Flöel, A. (2014). Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. Journal of Neuroscience, 34(23), 7862-7870.
[26] Ma, T., Tan, M. S., Yu, J. T., & Tan, L. (2014). Resveratrol as a therapeutic agent for Alzheimer’s disease. BioMed research international, 2014(1), 350516.
[27] Zhang, L. F., Yu, X. L., Ji, M., Liu, S. Y., Wu, X. L., Wang, Y. J., & Liu, R. T. (2018). Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food & function, 9(12), 6414-6426.
[28] Capiralla, H., Vingtdeux, V., Zhao, H., Sankowski, R., Al‐Abed, Y., Davies, P., & Marambaud, P. (2012). Resveratrol mitigates lipopolysaccharide‐and Aβ‐mediated microglial inflammation by inhibiting the TLR4/NF‐κB/STAT signaling cascade. Journal of neurochemistry, 120(3), 461-472.
[29] Lu, X., Ma, L., Ruan, L., Kong, Y., Mou, H., Zhang, Z., … & Le, Y. (2010). Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. Journal of neuroinflammation, 7, 1-14.
[30] Ruszkiewicz, J., & Albrecht, J. (2015). Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochemistry international, 88, 66-72.
[31] Kumar, A. P. S. N., Naidu, P. S., Seghal, N., & Padi, S. S. V. (2007). Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology, 79(1), 17-26.
[32] Howitz, K. T., Bitterman, K. J., Cohen, H. Y., Lamming, D. W., Lavu, S., Wood, J. G., … & Sinclair, D. A. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425(6954), 191-196.
[33] Wood, J. G., Rogina, B., Lavu, S., Howitz, K., Helfand, S. L., Tatar, M., & Sinclair, D. (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature, 430(7000), 686-689.
[34] Valenzano, D. R., Terzibasi, E., Genade, T., Cattaneo, A., Domenici, L., & Cellerino, A. (2006). Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Current biology, 16(3), 296-300.
[35] Baur, J. A., Pearson, K. J., Price, N. L., Jamieson, H. A., Lerin, C., Kalra, A., … & Sinclair, D. A. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444(7117), 337-342.
[36] V. Beljanski, “Resveratrol,” S. J. Enna and P. R. Bylund, Eds. New York: Elsevier, 2010, pp. 1–4
[37] Aggarwal, B. B., Bhardwaj, A., Aggarwal, R. S., Seeram, N. P., Shishodia, S., & Takada, Y. (2004). Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer research, 24(5A), 2783-2840.
[38] Yousef, M., Vlachogiannis, I. A., & Tsiani, E. (2017). Effects of resveratrol against lung cancer: In vitro and in vivo studies. Nutrients, 9(11), 1231.
[39] Ko, J. H., Sethi, G., Um, J. Y., Shanmugam, M. K., Arfuso, F., Kumar, A. P., … & Ahn, K. S. (2017). The role of resveratrol in cancer therapy. International journal of molecular sciences, 18(12), 2589.
[40] Chun, Y. J., Kim, M. Y., & Guengerich, F. P. (1999). Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochemical and biophysical research communications, 262(1), 20-24.
[41] Lagunas-Rangel, F. A., & Bermúdez-Cruz, R. M. (2020). Natural compounds that target DNA repair pathways and their therapeutic potential to counteract cancer cells. Frontiers in Oncology, 10, 598174.
[42] Berman, A. Y., Motechin, R. A., Wiesenfeld, M. Y., & Holz, M. K. (2017). The therapeutic potential of resveratrol: a review of clinical trials. NPJ precision oncology, 1(1), 35.
[43] Roy, S. K., Chen, Q., Fu, J., Shankar, S., & Srivastava, R. K. (2011). Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PloS one, 6(9), e25166.
[44] Tseng, S. H., Lin, S. M., Chen, J. C., Su, Y. H., Huang, H. Y., Chen, C. K., … & Chen, Y. (2004). Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clinical Cancer Research, 10(6), 2190-2202.
[45] Csaki, C., Mobasheri, A., & Shakibaei, M. (2009). Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis research & therapy, 11, 1-17.
[46] Siveen, K. S., Sikka, S., Surana, R., Dai, X., Zhang, J., Kumar, A. P., … & Bishayee, A. (2014). Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochimica et Biophysica Acta (BBA)-reviews on cancer, 1845(2), 136-154.
[48] Patel, K. R., Brown, V. A., Jones, D. J., Britton, R. G., Hemingway, D., Miller, A. S., … & Brown, K. (2010). Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer research, 70(19), 7392-7399.
[49] Howells, L. M., Berry, D. P., Elliott, P. J., Jacobson, E. W., Hoffmann, E., Hegarty, B., … & Gescher, A. J. (2011). Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer prevention research, 4(9), 1419-1425.
[50] Popat, R., Plesner, T., Davies, F., Cook, G., Cook, M., Elliott, P., … & Cavenagh, J. (2013). A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. British journal of haematology, 160(5).
[51] Moshawih, S., Mydin, R. B. S., Kalakotla, S., & Jarrar, Q. B. (2019). Potential application of resveratrol in nanocarriers against cancer: Overview and future trends. Journal of Drug Delivery Science and Technology, 53, 101187.
[52] Johnson, W. D., Morrissey, R. L., Usborne, A. L., Kapetanovic, I., Crowell, J. A., Muzzio, M., & McCormick, D. L. (2011). Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food and chemical toxicology, 49(12), 3319-3327.
[53] Gescher, A., Steward, W. P., & Brown, K. (2013). Resveratrol in the management of human cancer: how strong is the clinical evidence?. Annals of the New York Academy of Sciences, 1290(1), 12-20.
[54] Brown, V. A., Patel, K. R., Viskaduraki, M., Crowell, J. A., Perloff, M., Booth, T. D., … & Brenner, D. E. (2010). Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer research, 70(22), 9003-9011.
[55] Pace-Asciak, C. R., Hahn, S., Diamandis, E. P., Soleas, G., & Goldberg, D. M. (1995). The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clinica chimica acta, 235(2), 207-219.
[56] Chow, H. S., Garland, L. L., Hsu, C. H., Vining, D. R., Chew, W. M., Miller, J. A., … & Alberts, D. S. (2010). Resveratrol modulates drug-and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer prevention research, 3(9), 1168-1175.


