Exercise Mimetic Improves Memory In Old Mice
- This factor can be directly injected to restore cognition in animals.

Researchers have identified a platelet-derived factor that improves cognition in mice and published their findings in Nature Communications.
Finding the right molecules
This paper begins with the same refrain common in discussions about the biological effects of exercise: the inability of some older people to conduct exercise in any real capacity and therefore the need for drugs that mimic its effects. This includes its effects on the brain, as previous research has found that exercise causes new neurons to form [1] and improves memory and learning [2].
These effects have been attributed to exerkines, molecules that are released into the bloodstream as a result of exercise. Blood transfers between mice have demonstrated their effects [3]. Some research has identified specific organs, such as the liver [3] and muscle tissue [4], as being sources of these exerkines.
These researchers have previously identified platelets as a previously undiscovered source of exerkines, specifically the platelet-derived factor PF4 [5]. This paper builds upon their previous work by showing that it is indeed possible to use PF4 as an exercise mimetic in mouse models.
Testing the effects
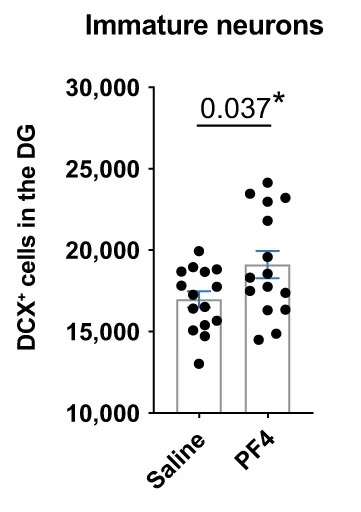
These researchers had previously found that administering PF4 directly to the brain has positive effects on neurogenesis [5]. To begin their experimentation, they chose to inject it into the tail veins of young adult mice, and their results were similarly positive. While it did not increase the number of neural precursor cells, it increased the number of immature neurons in the dentate gyrus, the part of the hippocampus responsible for memory formation. Therefore, the researchers reasoned that, instead of affecting stem cell proliferation, it may affect the survival of these cells and their differentiation into neurons.
As expected, RNA sequencing demonstrated that PF4 upregulated the genes responsible for neuronal differentiation and development. It was also found to be responsible for elongating the dentrites of young neurons. On the other hand, while exercise is known to positively affect synaptic plasticity in mature neurons, PF4 had no effect in this respect.
The researchers also performed a reverse experiment, finding out what happens to transgenic mice that do not produce any PF4 at all. These mice were mostly normal, except that they had significantly fewer proliferating neural precursors and differentiated neurons in the hippocampus compared to their wild-type counterparts. Other brain regions were not significantly affected. As they were unable to produce PF4, exercise did not help these modified mice produce more neurons.
Further experiments involving platelets and their deprivation demonstrated that platelets are responsible for creating PF4 during exercise. 18-month-old mice given antiplatelet serum along with an exercise regime did not benefit in the same way as exercising mice without the serum. Importantly, while young mice benefited within days, older mice needed to regularly run for a month in order to see these benefits.
The important question
Finally, the researchers tested the most important question: Does administering PF4 to aged mice improve their neurogenesis and memory?
The answer appeared to be yes. Similarly to the young mice, direct injection of PF4 to the tail veins of 20-month-old mice improved the number of neurons in the hippocampus. Further experiments showed that this neurogenesis had practical effects: older mice given PF4 had increases in memory, an increased willingness to explore new locations, and a stronger fear response to areas where they had experienced negative stimuli. The treated mice were significantly more able to learn new things.
If these results can be replicated in human beings through clinical trials, PF4 may become a clinically approved treatment for the decline in memory and learning abilities with aging.
Literature
[1] Van Praag, H., Kempermann, G., & Gage, F. H. (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience, 2(3), 266-270.
[2] Van Praag, H., Christie, B. R., Sejnowski, T. J., & Gage, F. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences, 96(23), 13427-13431.
[3] Horowitz, A. M., Fan, X., Bieri, G., Smith, L. K., Sanchez-Diaz, C. I., Schroer, A. B., … & Villeda, S. A. (2020). Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science, 369(6500), 167-173.
[4] Moon, H. Y., Becke, A., Berron, D., Becker, B., Sah, N., Benoni, G., … & van Praag, H. (2016). Running-induced systemic cathepsin B secretion is associated with memory function. Cell metabolism, 24(2), 332-340.
[5] Leiter, O., Seidemann, S., Overall, R. W., Ramasz, B., Rund, N., Schallenberg, S., … & Walker, T. L. (2019). Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem cell reports, 12(4), 667-679.