Air Pollution Promotes Cancer Through Inflammation
- Mutations do not appear to be directly caused by these particles.

Researchers have concluded that airborne fine particulate matter, which has been consistently linked to cancer, promotes lung cancer via inflammation and not necessarily via mutagenesis [1].
Not just mutations
Common wisdom says that carcinogens increase cancer risk mostly by promoting oncogenic mutations. Research has reported that this is true for some carcinogens, such as tobacco smoke [2], and more recently, red meat [3]. In both cases, the researchers were able to identify carcinogen-specific mutational signatures.
However, there is a growing understanding that while mutations are necessary for the emergence of cancer, various factors, including environmental ones, can promote it via alternative pathways. In this new study, a group of researchers set out to investigate whether airborne particulate matter can trigger and exacerbate lung cancer even without directly causing mutations.
More cancer in polluted areas
Particulate matter with a particle size of 2.5 microns or less (PM2.5) is highly invasive and has been linked by previous research to various adverse health effects [4]. The scientists started by analyzing existing data to establish a link between PM2.5 levels and the incidence of lung cancer in three regions.
To correct for the robust effect of smoking, the scientists focused on the subtype of lung cancer promoted by mutations in the gene EGFR, since this subtype frequently arises in non-smokers. While smoking is a mighty risk factor in lung cancer, 10% to 20% of all lung cancer cases in the US occur in people who have never smoked. Additionally, 8% of lung adenocarcinomas in smokers lack smoking-related mutations: they are caused by factors other than smoking [5].
The analysis found strong correlations between EGFR-related lung cancer and mean PM2.5 levels in England, South Korea, and Taiwan (left to right):
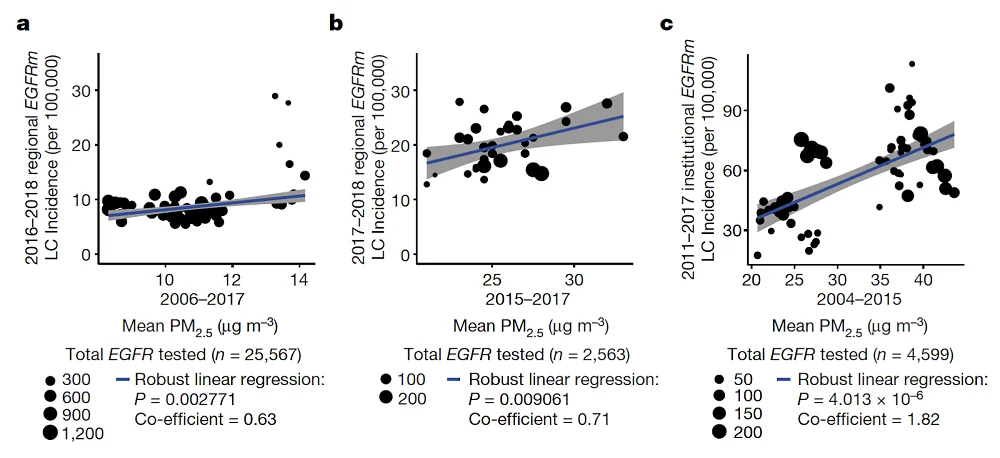
Using a fourth, smaller cohort of Canadian women that included personal migration history, the researchers determined that the frequency of EGFR-driven lung cancer became significantly higher after three years of living in an area with high PM2.5 levels.
Particulate matter drives lung cancer in mice
The researchers then used genetically engineered mouse models of lung adenocarcinoma to understand how exposure to particulate matter promotes its development. These models were created by injecting mice with a viral vector carrying a human EGFR gene with a certain oncogenic mutation. The mice were then subjected to physiologically relevant doses of PM2.5. Ten weeks later, the PM2.5 group showed a larger number of early tumors compared to controls in a dose-dependent manner. Similar trend emerged when exposing mice to PM2.5 before inducing the viral vector, which shows that PM2.5 can “prime” the organism for accelerated cancer development even before the appearance of an oncogenic mutation.
Interestingly, similar results were obtained when injecting mice with another well-known lung cancer-causing mutation in the KRAS gene. The effect was more pronounced 10 weeks after the exposure to PM2.5 than 3 weeks after, showing that PM2.5 triggers a lingering mechanism that promotes early tumorigenesis that does not involve mutagenesis, as the exposure to PM2.5 did not increase the mutation burden).
Increased inflammatory response
The researchers hypothesized that increased tumorigenesis might be linked to immune response. Indeed, when they introduced the mutated EGFR gene into immunocompromised mice that lacked B, T, and natural killer cells, this did not result in increased tumorigenesis. Conversely, in immunocompetent mice the acceleration of tumorigenesis was accompanied by both acute and long-term immune reaction caused by macrophage infiltration.
The researchers found that those macrophages secreted large amounts of the pro-inflammatory cytokine IL-1β. Since treatment with an anti-IL-1β antibody was sufficient to significantly slow tumorigenesis, the authors suggest that PM exposure drives tumorigenesis via increased macrophage-mediated inflammation. Prof. Charles Swanton, the study’s lead investigator, said in a statement:
Cells with cancer-causing mutations accumulate naturally as we age, but they are normally inactive. We’ve demonstrated that air pollution wakes these cells up in the lungs, encouraging them to grow and potentially form tumors. The mechanism we’ve identified could ultimately help us to find better ways to prevent and treat lung cancer in never-smokers. If we can stop cells from growing in response to air pollution, we can reduce the risk of lung cancer.
Conclusion
The study suggests that air pollution (and probably some other carcinogens) drive tumorigenesis at least in part by increasing inflammation. This finding is important since while we might not be able to completely avoid contact with carcinogens, we have ways to mitigate inflammation, for instance via diet or lifelong exercise [6].
Literature
[1] Hill, W., Lim, E. L., Weeden, C. E., Lee, C., Augustine, M., Chen, K., … & Swanton, C. (2023). Lung adenocarcinoma promotion by air pollutants. Nature, 616(7955), 159-167.
[2] Alexandrov, L. B., Ju, Y. S., Haase, K., Van Loo, P., Martincorena, I., Nik-Zainal, S., … & Stratton, M. R. (2016). Mutational signatures associated with tobacco smoking in human cancer. Science, 354(6312), 618-622.
[3] Gurjao, C., Zhong, R., Haruki, K., Li, Y. Y., Spurr, L. F., Lee-Six, H., … & Giannakis, M. (2021). Discovery and features of an alkylating signature in colorectal cancer. Cancer discovery, 11(10), 2446.
[4] Li, R., Zhou, R., & Zhang, J. (2018). Function of PM2. 5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncology letters, 15(5), 7506-7514.
[5] Jamal-Hanjani, M. (2023). The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature.
[6] Nilsson, M. I., Bourgeois, J. M., Nederveen, J. P., Leite, M. R., Hettinga, B. P., Bujak, A. L., … & Tarnopolsky, M. A. (2019). Lifelong aerobic exercise protects against inflammaging and cancer. PLoS One, 14(1), e0210863.







