Extending Monkeys’ Reproductive Span With Stem Cells
- One healthy monkey was naturally conceived and born after the treatment.

An investigation into transplanting human embryonic stem cells (hESC)-derived MSC-like cells (M cells) into the ovaries of cynomolgus monkeys suggests an extension of female reproductive span accompanied by a reduction in senescence-associated processes, such as inflammation, fibrosis, oxidative damage, and apoptosis [1].
Increasing healthspan by delaying menopause
Aging of the female reproductive system precedes aging of other systems, resulting in women living one-third of their lives after menopause [2]. Menopause results not only in the cessation of reproduction but is also associated with health problems such as osteoporosis [3], cardiovascular problems [4], and neurodegenerative diseases [5]. Therefore, delaying menopause could allow women to live longer, disease-free lives, and so the authors chose stem cell transplantation as a possible therapeutic approach to delay ovarian aging and increase the reproductive span.
Perimenopausal Chinese women’s ovarian reserve
Female reproductive aging is tightly linked to ovarian reserve, which is measured by the number of primordial follicles. The ovarian follicle is a cellular structure that releases an egg that can be fertilized. The follicle’s oocyte is surrounded by granulosa cells (GCs) and theca cells (TCs).
A female’s ovarian reserve is established while still in her mother’s womb. Once a woman enters puberty, every menstrual cycle draws from the reserve of primordial follicles. This process slowly diminished the ovarian reserve, leading to menopause.
Multiple studies have analyzed ovarian reserve decline. However, none has analyzed the ovarian reserve of Asian women. Since there is variability among different demographics regarding the age at menopause, there are also likely to be differences in ovarian reserve decline among different demographic groups. To address that, those researchers analyzed the ovarian reserve in Chinese females.
The researchers collected 28 ovaries from 26 Chinese women between 35 and 52 years old and counted the follicles in the stained, thin ovarian sections.
In the youngest group of females, aged 35-39, the average number of primordial follicles per ovary was 11,098. This number decreased with age. In females aged 40-44, it was 6,728; in the 45-49 group, it was 1,019, and there were only 151 in the oldest group aged 50-52. On the other side, the diameters of oocytes and oocyte nuclei of primordial follicles were similar between females of different ages.
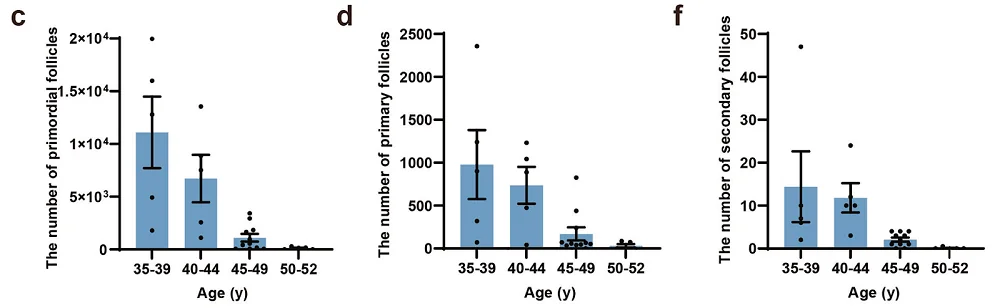
The authors also observed the primordial follicles to develop into primary and secondary follicles in perimenopausal women. However, the number of both decreased with age. They conclude that the primordial follicles in perimenopausal women could still develop into growing follicles.
Alleviating ovarian aging with stem cells
Mesenchymal stem cell (MSC)-based therapy has shown potential in reversing ovarian aging and recovering fertility in animal models and women suffering from premature ovarian insufficiency [6-9]. However, this approach has some limitations.
hESC-derived M cells resemble MSCs but can overcome some of MSCs’ limitations, such as manufacturing at scale. Research has also discovered that they also have more potent immunomodulatory and anti-fibrotic functions [10].
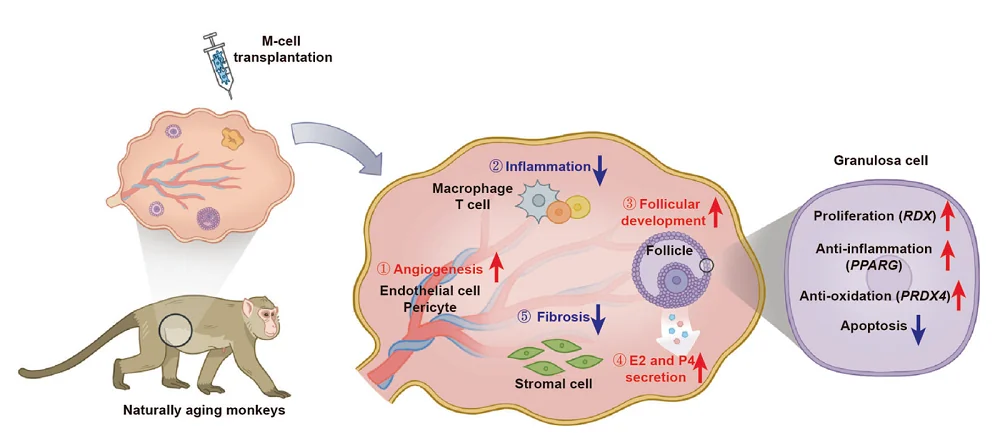
With the aim of testing “the safety and efficacy of M-cell transplantation in ameliorating human physiological ovarian aging,” the researchers have chosen naturally aging cynomolgus monkeys as their model systems, since ovarian aging shares plenty of similarities between monkeys and humans.
The authors selected ten perimenopausal monkeys and divided them into two groups: three as controls and seven in the treatment group. They injected the monkeys’ ovaries with M cells twice, one month apart, and followed up for eight months. The results suggested the safety of the treatment, as no acute inflammation or malignant diseases were observed.
A comparison of treated and untreated monkeys showed some positive impacts of the treatment. The researchers observed significantly larger ovarian diameters and thicker endometria in the treated group. Sex hormone levels were also positively impacted – estradiol levels were higher than control in 6 and 8 months post-treatment check-ups and progesterone remained at higher levels in treated monkeys, while in the control group, progesterone levels decreased during the follow-up period.
The researchers also tested the impact of M-cell treatment on follicle development. Assessment of the number of follicles showed increased numbers of growing follicles in the treated group compared to the control group, suggesting increased fertility potential following the treatment.
The treatment also alleviated ovarian aging, as the examination of ovaries showed decreased fibrosis, higher numbers of proliferative GC cells, which are essential for follicle development, and reduced DNA damage markers in GC cells in the treated group compared to the control.
Extending reproductive span
While the changes in molecular processes and hormonal levels are important for testing ovarian aging, the ultimate test of whether the treatment works is whether the monkeys can conceive a child.
In the initial test, the researchers injected monkeys with recombinant hormones to stimulate follicle growth and egg production. Two monkeys from the control group that were injected with hormones didn’t yield oocytes. Four monkeys from the treatment group injected with hormones yielded between 1 and 33 oocytes. Two monkeys produced mature oocytes that were collected for intracytoplasmic sperm injection (ICSI) and successfully fertilized. Out of those, two fertilized eggs developed to the blastocyst stage.
Monkeys were also allowed to breed for two months to test the possibility of natural conception. One treated monkey got pregnant and delivered a healthy, full-term baby. The baby is around three years old and still healthy, similar to the babies delivered by younger monkeys.
Molecular mechanism
The molecular mechanism behind the recovery of ovarian function following M-cell transplantation was also studied. The researchers collected ovaries from two control and three treated monkeys and measured their gene expression. They also employed a wide array of experimental tools, including cell culture-based assays, and gene inactivation experiments that allowed them to find the molecular processes that play a role in the impact of M cells on ovarian aging.
Their results suggest that M-cell therapy led to a decrease in inflammation, fibrosis, oxidative damage, and apoptosis. It also promoted follicle development by increasing cell proliferation, angiogenesis, and hormone response levels in perimenopausal ovaries.
The researchers concluded that their results show the feasibility of using M-cell transplantation to alleviate ovarian aging and the possibility of extending reproductive lifespan, but more research is necessary to establish safety and efficacy in humans.
Literature
[1] Yan, L., Tu, W., Zhao, X., Wan, H., Wu, J., Zhao, Y., Wu, J., Sun, Y., Zhu, L., Qin, Y., Hu, L., Yang, H., Ke, Q., Zhang, W., Luo, W., Xiao, Z., Chen, X., Wu, Q., He, B., Teng, M., … Wang, H. (2024). Stem cell transplantation extends the reproductive life span of naturally aging cynomolgus monkeys. Cell discovery, 10(1), 111.
[2] Lobo, R. A., & Gompel, A. (2022). Management of menopause: a view towards prevention. The lancet. Diabetes & endocrinology, 10(6), 457–470.
[3] Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K., Harada, Y., Azuma, Y., Krust, A., Yamamoto, Y., Nishina, H., Takeda, S., Takayanagi, H., Metzger, D., Kanno, J., Takaoka, K., Martin, T. J., Chambon, P., & Kato, S. (2007). Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell, 130(5), 811–823.
[4] Zhu, D., Chung, H. F., Dobson, A. J., Pandeya, N., Giles, G. G., Bruinsma, F., Brunner, E. J., Kuh, D., Hardy, R., Avis, N. E., Gold, E. B., Derby, C. A., Matthews, K. A., Cade, J. E., Greenwood, D. C., Demakakos, P., Brown, D. E., Sievert, L. L., Anderson, D., Hayashi, K., … Mishra, G. D. (2019). Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. The Lancet. Public health, 4(11), e553–e564.
[5] Xiong, J., Kang, S. S., Wang, Z., Liu, X., Kuo, T. C., Korkmaz, F., Padilla, A., Miyashita, S., Chan, P., Zhang, Z., Katsel, P., Burgess, J., Gumerova, A., Ievleva, K., Sant, D., Yu, S. P., Muradova, V., Frolinger, T., Lizneva, D., Iqbal, J., … Ye, K. (2022). FSH blockade improves cognition in mice with Alzheimer’s disease. Nature, 603(7901), 470–476.
[6] Zhao, Y., Ma, J., Yi, P., Wu, J., Zhao, F., Tu, W., Liu, W., Li, T., Deng, Y., Hao, J., Wang, H., & Yan, L. (2020). Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem cell research & therapy, 11(1), 466.
[7] Yan, L., Wu, Y., Li, L., Wu, J., Zhao, F., Gao, Z., Liu, W., Li, T., Fan, Y., Hao, J., Liu, J., & Wang, H. (2020). Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell proliferation, 53(12), e12938.
[8] Umer, A., Khan, N., Greene, D. L., Habiba, U. E., Shamim, S., & Khayam, A. U. (2023). The Therapeutic Potential of Human Umbilical Cord Derived Mesenchymal Stem Cells for the Treatment of Premature Ovarian Failure. Stem cell reviews and reports, 19(3), 651–666.
[9] Tian, C., He, J., An, Y., Yang, Z., Yan, D., Pan, H., Lv, G., Li, Y., Wang, Y., Yang, Y., Zhu, G., He, Z., Zhu, X., & Pan, X. (2021). Bone marrow mesenchymal stem cells derived from juvenile macaques reversed ovarian ageing in elderly macaques. Stem cell research & therapy, 12(1), 460.
[10] Wu, J., Song, D., Li, Z., Guo, B., Xiao, Y., Liu, W., Liang, L., Feng, C., Gao, T., Chen, Y., Li, Y., Wang, Z., Wen, J., Yang, S., Liu, P., Wang, L., Wang, Y., Peng, L., Stacey, G. N., Hu, Z., … Hao, J. (2020). Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell research, 30(9), 794–809.








