Restoring the Strength of Natural Killer Cells
- These cells lose their fundamental potency with age.
- Natural killer cells lose their binding and toxicity abilities with aging.
- This is due to an overabundance of the protein Cdc42, which disrupts their internal organization.
- Inhibiting Cdc42 restores some of their abilities, allowing them to perform more like younger cells.
In Aging Cell, researchers have described why older natural killer (NK) cells lose their ability to eliminate harmful cells and a potential treatment for this decline.
Judgment and ability
At the cellular level, there is no due process. Natural killer (NK) cells judge other cells’ guilt or innocence by their surface proteins. They ruthlessly exterminate any foreign cells they find, which is what causes organ rejection; when they mistakenly attack the body’s own functional cells, autoimmune disorders are the result.
Even when uninfected and native to the body, cells can be guilty of two severe crimes: cancer and senescence. Encouraging NK cells to attack cancer despite its protections is a core part of modern oncology, and senescent cells are able to evade immune clearance as well [1].
However, errors in judgment are not the only potential issues with NK cells. This paper focuses on the armament of these cells, investigating the age-related reduction of their ability to do their jobs at all.
Older cells are much weaker
In their first experiment, the researchers derived NK cells from old groups of approximately 70-year-old humans and 700-day-old mice alongside young groups of approximately 21-year-old humans and 100-day-old mice. The human cells were tested against four groups of human dermal fibroblasts: three that had been driven senescent through toxicity, replication, or radiation, and a fourth that was derived from 75-year-old people; the mouse cells were tested against similar murine counterparts.
The results were entirely unsurprising. In every case, particularly against the naturally aged cells, the younger NK cells were far more effective in killing senescent fibroblasts than their older counterparts.
The differences were obvious even under a microscope. Young human NK cells were able to rapidly and tightly bind to senescent cells, killing them quickly, and then rapidly move on to the next senescent cell. Older NK cells failed at both; they were unable to form tight bonds, and they were lethargic in moving on to the next target.
Testing against various cancer cell lines yielded similar results. Older NK cells were less effective against multiple varieties of lymphoma and leukemia. Like their interactions with the senescent cells, this was found to be due to a lack of conjugation; the older NK cells were simply less able to bind with and properly destroy the cancer cells. An investigation into targeting mechanisms found that recognition of defective cells was not the reason why the older NK cells were less able to attack.
Instead, the older cells were found to have issues with their fundamental cytotoxic machinery. Normally, an NK cell will bind to a target cell and then attack it with a combination of perforin, which penetrates the cell, and granzyme B, which kills it. The attack itself requires granules of these weapons to be released in degranulation, and older NK cells were found to have both less binding ability and less degranulation than younger cells.
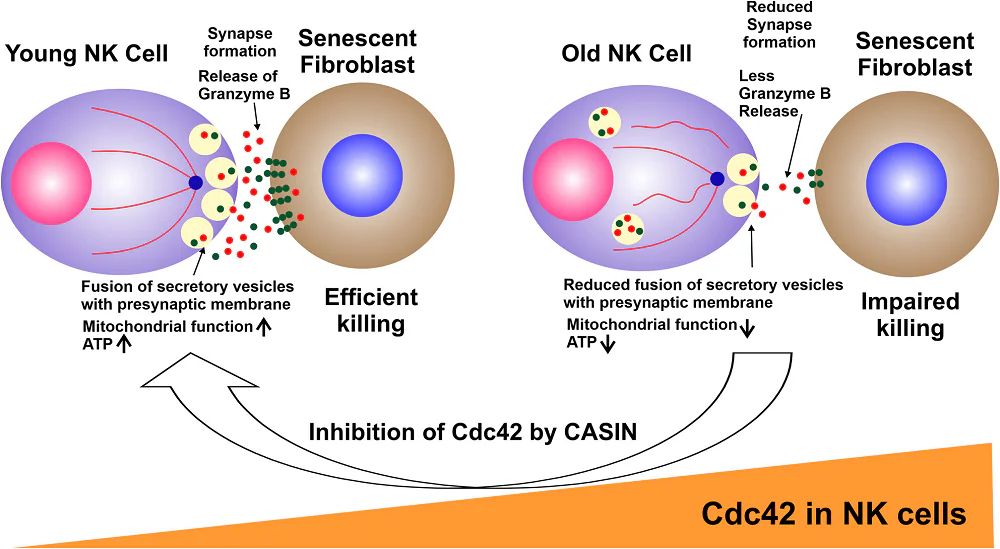
These reductions in ability were confirmed with a gene expression analysis. As expected, the older cells had downregulations in metabolism, activation, and core processes responsible for membrane transport and degranulation; the older cells were simply less able to bring their weapons to bear.
A potential solution
The researchers noted a key protein that was upregulated in their analysis: Cdc42, which has been noted to affect microtubular organization and increases with age. Previous work has found that Cdc42 has been implicated in the aging of hematopoietic stem cells (HSCs) [3], and proper microtubular organization is critical in correctly polarizing NKs and allowing cytotoxic granules to get to where they need to be.
A closer look at these cells’ microtubules suggested that this is likely to be the key issue. Older NK cells were significantly less organized than younger cells; in younger cells, Cdc42 sits on one side of the cell while tubulin sits on the other, but older cells did not have this polarity. Exposing older NK cells to CASIN, an inhibitor of Cdc42, was found to be successful in helping the older cells restore this balance.
Older NK cells exposed to CASIN received benefits in both conjugation and degranulation; in conjugation, the CASIN-exposed older cells even appeared to be slightly stronger than the younger ones. There were also benefits for mitochondria as well; older cells exposed to CASIN had even more of the energy transfer molecule ATP than their younger counterparts did, potentially further bolstering their overall ability.
However, CASIN did not give perfect results. While being substantially better than the unexposed older cells, the CASIN-treated older cells were not nearly as able to kill as many leukemic or senescent cells as younger cells were. Compared to untreated older and younger mice, CASIN-treated older mice were roughly halfway between those groups in their ability to remove senescent cells in the bone marrow and the spleen. CASIN was found to only affect the ability of NK cells and did not affect their proliferation.
These results, while substantially beneficial, were still only done in mice, and the potential side effects of using CASIN or another Cdc42 inhibitor in human beings have not been eludicated. The researchers suggest that further work should be done in exploring this approach as a treatment for age-related diseases that involve cancer or senescence.
Literature
[1] Pereira, B. I., Devine, O. P., Vukmanovic-Stejic, M., Chambers, E. S., Subramanian, P., Patel, N., … & Akbar, A. N. (2019). Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nature communications, 10(1), 2387.
[2] Topham, N. J., & Hewitt, E. W. (2009). Natural killer cell cytotoxicity: how do they pull the trigger? Immunology, 128(1), 7-15.
[3] Florian, M. C., Dörr, K., Niebel, A., Daria, D., Schrezenmeier, H., Rojewski, M., … & Geiger, H. (2012). Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell stem cell, 10(5), 520-530.