Investigating Why Brain Plasticity Decreases with Age
- A key compound increases with stress but decreases with age.

Publishing in Aging, a team of researchers has used a rat model to investigate a possible reason why old people are less able to learn new things.
A critical receptor
This paper begins with a discussion of receptors for N-methyl-D-aspartate (NMDA), a compound that affects multiple aspects of cognition, most notably neuroplasticity: the brain’s ability to learn new things [1]. NMDA function is well-known to decline with age, and a substantial amount of research has been conducted on the relationship to overall brain function [2].
To be activated, the NMDA receptor requires the binding of two separate compounds: the common amino acid glutamate and, principally, the more specific agonist D-serine [3]. D-serine is formed from the conversion of L-serine through serine racemase (SR) [4], a compound that is secreted by the neuroglia. Interestingly, this secretion can be heightened under stress conditions, such as injury [5] and the presence of amyloid precursors [6].
How SR production declines with age
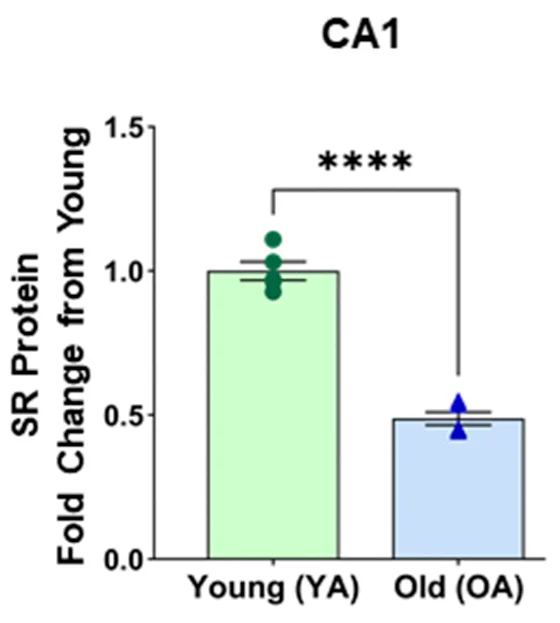
These researchers used rat models to take a closer look at SR. First, they examined the brains of Fischer 344 rats, a standard breed of wild-type animals. 26-month-old rats, as expected, had significantly less SR than 5-month-old rats throughout the prefrontal cortex, a brain region critical for learning and cognition, as well as the hippocampus, which is responsible for memory. While the effects were significant in both male and female rats, the effects were exceptionally strong in the CA1, a specific part of the hippocampus, in male rats.
The researchers also discovered a potential measurement issue. β-actin is a common protein that is often used as a reference with which to measure other proteins. However, β-actin is also different between older males and older females in the prefrontal cortex.
As these researchers have previously used a viral vector to improve cognition in middle-aged rats by upregulating SR [7], these results shed more light on what age-related changes could potentially be reversed by such a treatment. As male and female rats express SR differently, it would be crucial to take sex differences into account if a clinical trial for increasing SR is performed on human beings.
Literature
[1] Zorumski, C. F., & Izumi, Y. (2012). NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews, 36(3), 989-1000.
[2] Foster, T. C. (2012). Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Progress in neurobiology, 96(3), 283-303.
[3] Papouin, T., Ladépêche, L., Ruel, J., Sacchi, S., Labasque, M., Hanini, M., … & Oliet, S. H. (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell, 150(3), 633-646.
[4] Wolosker, H., Blackshaw, S., & Snyder, S. H. (1999). Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences, 96(23), 13409-13414.
[5] Perez, E. J., Tapanes, S. A., Loris, Z. B., Balu, D. T., Sick, T. J., Coyle, J. T., & Liebl, D. J. (2017). Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. The Journal of Clinical Investigation, 127(8), 3114-3125.
[6] Wu, S., Basile, A. S., & Barger, S. W. (2007). Induction of serine racemase expression and D-serine release from microglia by secreted amyloid precursor protein (sAPP). Current Alzheimer Research, 4(3), 243-251.
[7] Yegla, B., Rani, A., & Kumar, A. (2023). Viral vector-mediated upregulation of serine racemase expression in medial prefrontal cortex improves learning and synaptic function in middle age rats. Aging (Albany NY), 15(7), 2433.








