Pre-Senescent Cells May Be Damaging and Treatable
- Young cells secrete factors just as old cells do.

Researchers have described an intermediary state between senescent and young cells, the inflammatory problems caused by these cells, and how young cells secrete a protein that may alleviate these problems.
What happens before senescence?

Read More
As these researchers note, the problems with senescent cells are very well-known and thoroughly documented. However, despite this, senescent cells are not the only older cells in the tissues of older people; many more cells are on their way to senescence. The researchers have termed these pre-senescent cells “mid-old” cells.
A difference in inflammation
This experiment began by culturing human fibroblasts in vitro and observed their chemical changes over time. They divided the cells into three groups: young cells (1-2 days old) that had almost none of the senescence marker SA-ß-gal, mid-old cells (5-7 days old) that still had very little SA-ß-gal, and fully senescent cells over two weeks old that were heavily infused with SA-ß-gal.
In most cases, mid-old fibroblasts behaved much like younger fibroblasts. They retained their ability to respond to external stimuli, which senescent fibroblasts largely cannot do. The researchers hold that this is because mid-old cells have only slight decreases in the necessary signaling proteins. Mid-old cells could also still proliferate, although with slightly decreased ability compared to younger cells.
Of the well-known senescence markers p16, p21, and p53, there was a clear distinction: like with SA-ß-gal, mid-old cels produced slightly more but fully senescent cells produced far more. Some, but not all, inflammatory and anti-inflammatory cytokines also followed a relatively smooth pattern; senescent cells produce some of both in greater and lesser quantities than younger cells, and in most cases, the mid-old cells’ production is only slightly deviated in the older cells’ direction.
Gene expression analysis confirmed this overall pattern. In general, mid-old cells have much more in common with young cells than with senescent cells.
This was not true, however, for the inflammatory cytokine IL-1ß, which has broad-spectrum effects, particularly relating to inflammatory diseases [1]. Senescent fibroblasts produce more than twice as much IL-1ß as young fibroblasts. However, mid-old cells were producing roughly twice as much as the senescent fibroblasts were. The researchers singled out the acute response protein SAA1, which is known to drive inflammation [2] and muscle atrophy [3], as the driving factor behind this change.
Tissue analysis shows a sharp difference
With these differences in hand, the researchers then examined donated human tissues. They found that elderly people had far more mid-old cells than young people did. While this was not true for epithelial tissues, whose cells are rapidly replaced, fibroblast-rich tissues, such as the colon and lung, were found to have far more IL-1ß and SAA1 – just as they were in mice. Smooth muscle tissues in humans also have these mid-old signifiers substantially upregulated.
The researchers posit that this increase, along with a decrease in anti-inflammatory cytokines such as CXCL12, is a substantial part of why inflammation is such a large problem for older people.
This increase in SAA1 was also hypothesized to harm the structures of organs. The extracellular matrix, particularly the basement membrane, is required for proper function, even in epithelial tissues. An increase in MMP9, which occurs in mid-old and old cells and is driven by SAA1 [4], harms this membrane [5]. An examination of older tissues, along with an in vitro experiment, confirmed this hypothesis: preventing mid-old cells from expressing MMP9 also prevented collagen degradation.
The anti-SASP
The researchers then attempted to rescue these mid-old cells by co-culturing them with young cells. This rescued many of the mid-old cells’ functions, including better proliferation. This experiment also showed a tendency towards reduced inflammation, particularly in the heavily upregulated IL-1ß. This, the researchers believe, demonstrates the existence of a secretory phenotype that is the functional opposite of the SASP: the Juvenile-Associated Secretory Phenotype (JASP). Further experiments revealed that the anti-inflammatory cytokine SLIT2, which is secreted by young cells, is a significant part of the JASP.
This line of inquiry was continued. The researchers injected 23-month-old mice, nearly at the end of their lifespans, with a recombinant murine variant of SLIT2. After a month of treatment, the SLIT2 group was found to have considerably larger muscles and could run notably faster than the control group.
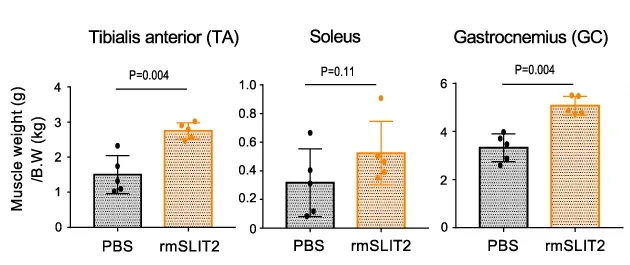
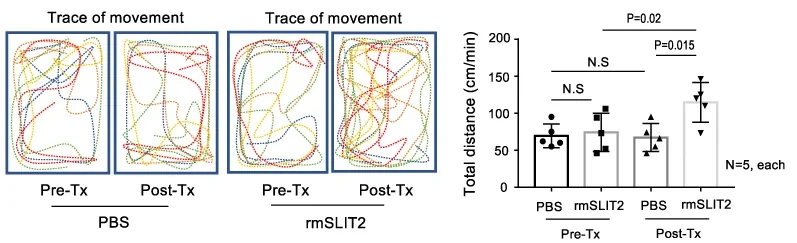
As usual, these results were found in cultured cells and in mice. However, if SLIT2 can mitigate the damage that mid-old cells have been found to cause, it may be appropriate to conduct human clinical trials of this protein to test it against sarcopenia or other muscle- or tissue-related ailments.
Literature
[1] Kaneko, N., Kurata, M., Yamamoto, T., Morikawa, S., & Masumoto, J. (2019). The role of interleukin-1 in general pathology. Inflammation and regeneration, 39, 1-16.
[2] Jensen, L. E., & WHITEHEAD, A. S. (1998). Regulation of serum amyloid A protein expression during the acute-phase response. Biochemical Journal, 334(3), 489-503.
[3] Langhans, C., Weber-Carstens, S., Schmidt, F., Hamati, J., Kny, M., Zhu, X., … & Fielitz, J. (2014). Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PloS one, 9(3), e92048.
[4] Lee, H. Y., Kim, M. K., Park, K. S., Bae, Y. H., Yun, J., Park, J. I., … & Bae, Y. S. (2005). Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochemical and biophysical research communications, 330(3), 989-998.
[5] Zeng, Z. S., Cohen, A. M., & Guillem, J. G. (1999). Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis, 20(5), 749-755.








