Precision Targeting of Senescent Cells
- Soap bubbles may be less toxic than sand grains.
In a journal called Small, researchers have described a new targeting mechanism for delivering senolytic compounds where they need to go.
Finding the right nanoparticle

Read More
This paper begins with a discussion of the well-known features of cellular senescence and laments that, despite all the work done in this area, no senolytic has yet been approved for clinical use. The researchers provide evidence that this is due to both efficacy and targeting: senolytics do not always solely affect senescent cells [1].
Previous work has focused on using galactose as a carrier for such potential drugs [2], as senescent cells are characterized by the presence of SA-β-gal, a compound that naturally cleaves galactose. This approach has, in early studies, been found to reduce the toxicity of navitoclax, the senolytic that is the focus of this study [1].
However, much of that previous work was focused on encapsulating porous silica with galactose as a nanocarrier for the drug, and these researchers note that porous silica can be toxic [3]. Trying to directly modify drugs with galactose changes is also not perfect, as this process changes their structure and is difficult to accomplish [4].
The soap approach
Instead of silica, these researchers chose to encapsulate their drug in amphiphilic micelles, which are very similar to soap bubbles and have been previously examined in drug delivery [5]. Here, the micelles have the water-attracted (hydrophilic) portion facing outwards and holding the galactose, with the water-repellent (hydrophobic) portion facing inwards to contain the navitoclax. The researchers go into detail regarding the chemistry of how they accomplished this, using a variety of branches extending from the central component and then assembling those molecules together to form a bubble.
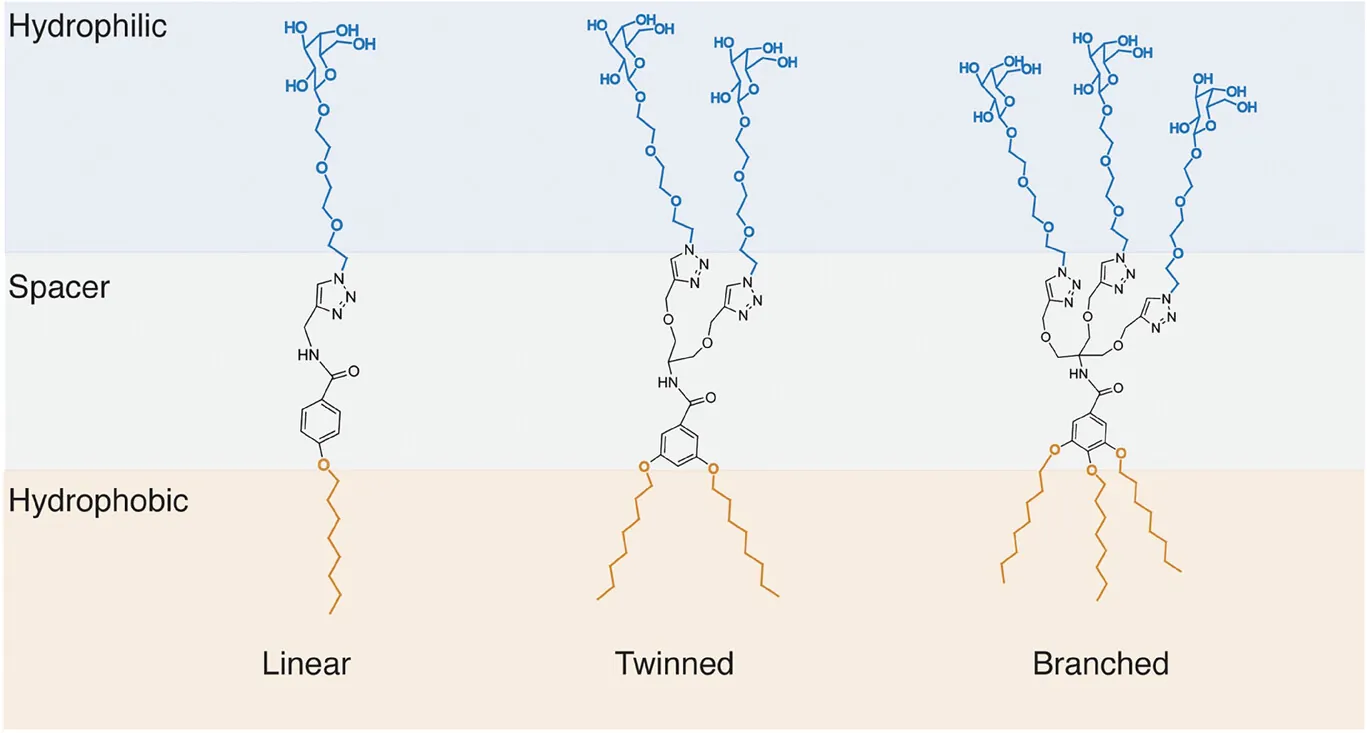
Of these three approaches, the branched variant was found to be the most effective, as shown by an in vitro test using fluorescent Nile Red dye. Furthermore, the bubble was found to be protective: in the absence of β-galactosidase, only 6% of the total fluorescence was reduced 24 hours after exposure, while in its presence, 50% of it was gone within 6 hours and 90% within 24 hours.
Effective in cells
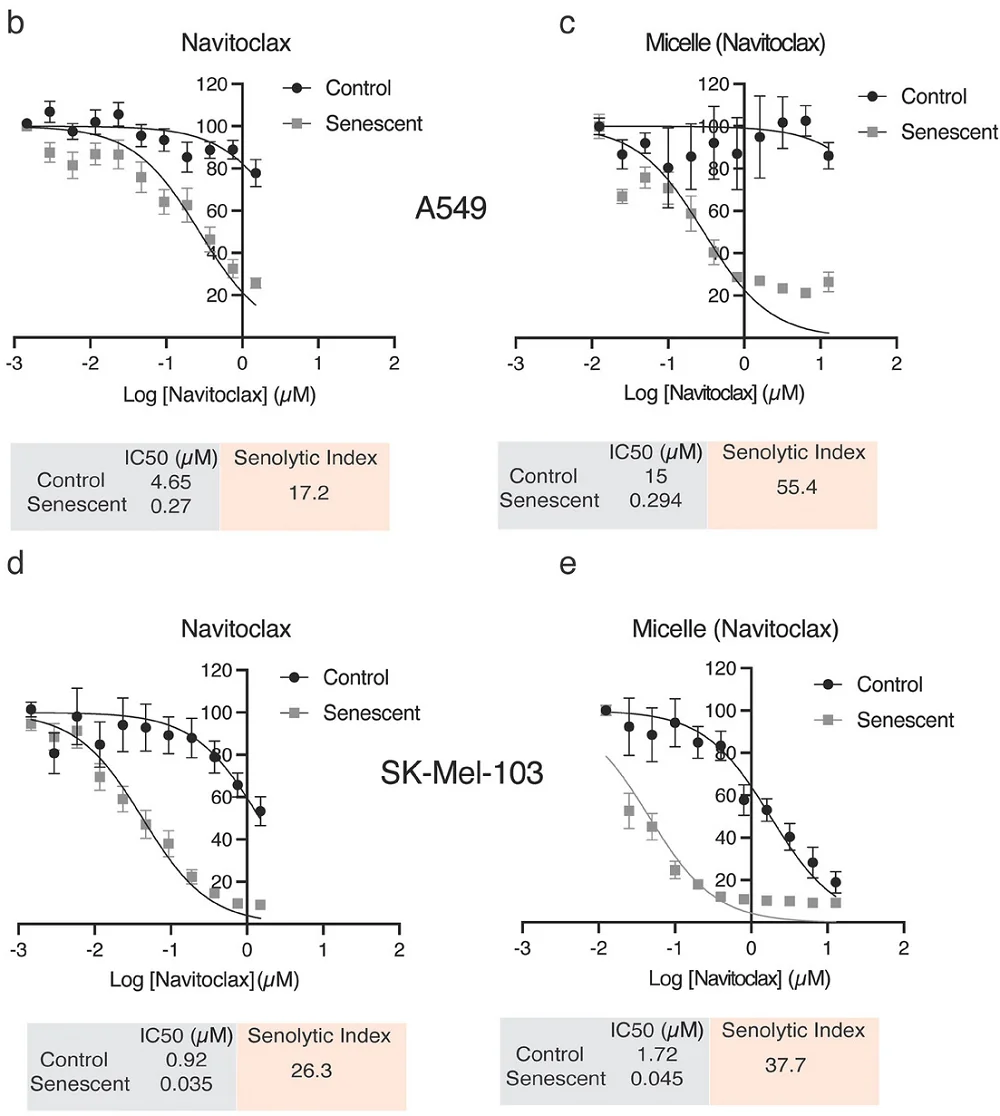
The researchers then tested their compound against actual senescent cells, specifically cells derived from lung cancer (A549) and melanoma (SK-MEL-103) lines. The senolytic index, which measures efficacy versus off-target effects, was much stronger in the encapsulated variant versus raw navitoclax alone: the micelle-encapsulated drug was more selective against senescent cells, particularly in the A549 line.
However, this paper is only a cellular study, and there were no animals involved. Furthermore, the experiments were conducted solely on cell lines derived from cancer, and it has yet to be experimentally determined how other cells or living organisms might respond to these micelles in the body. Still, this paper serves as a useful proof of concept, explaining how a drug can be targeted to the cells that need it most.
Literature
[1] González‐Gualda, E., Pàez‐Ribes, M., Lozano‐Torres, B., Macias, D., Wilson III, J. R., González‐López, C., … & Muñoz‐Espín, D. (2020). Galacto‐conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging cell, 19(4), e13142.
[2] Muñoz‐Espín, D., Rovira, M., Galiana, I., Giménez, C., Lozano‐Torres, B., Paez‐Ribes, M., … & Serrano, M. (2018). A versatile drug delivery system targeting senescent cells. EMBO molecular medicine, 10(9), e9355.
[3] Lin, Y. S., & Haynes, C. L. (2010). Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. Journal of the American Chemical Society, 132(13), 4834-4842.
[4] Guerrero, A., Guiho, R., Herranz, N., Uren, A., Withers, D. J., Martínez‐Barbera, J. P., … & Gil, J. (2020). Galactose‐modified duocarmycin prodrugs as senolytics. Aging Cell, 19(4), e13133.
[5] Parshad, B., Prasad, S., Bhatia, S., Mittal, A., Pan, Y., Mishra, P. K., … & Fruk, L. (2020). Non-ionic small amphiphile based nanostructures for biomedical applications. RSC advances, 10(69), 42098-42115.







