Fighting Inflammation May Cause More Senescence
- Senescent cells protect themselves from the immune system.
A researcher publishing in Biogerontology has reviewed the literature on the relationship between cellular senescence and the immune system, finding that dwindling immune surveillance allows senescent cells to accumulate.
The immune system needs to be deadly
On the cellular level, the bodies of vertebrates are brutal police states. Immune cells report, attack, and kill anything that gives off improper chemical signals, which includes the body’s own cells. When immune cells go after healthy, functioning cells, this is a potentially fatal immune disorder; however, when they are convinced not to attack unhealthy cells, such as in tumors, this can lead to the spread of cancer.

Read More
Senescent cells are also frequently policed and destroyed by the immune system. However, with aging, this ability declines, which is part of why senescent cells accumulate in older people [1]. While senescent cells and their SASP are often associated with inflammation, there is an increase in anti-inflammatory activity as well; for example, senescent cells in mice have been found to greatly suppress immune activity in a way that leads to the development of cancer [2].
Much of this anti-immune activity originates from immunosuppressive cells, such as Tregs, myeloid-derived suppressor cells (MDSCs), and healing-polarized M2 macrophages. Polarizing macrophages away from the M1 inflammatory type and towards healing is normally considered a good thing in the context of systemic inflammation in aging (inflammaging), but systemic immunosuppression has been reported to make inflammaging worse [1].
Senescent cells, themselves, also secrete compounds that convince immune cells not to destroy them. Cytokines, which are often secreted to promote inflammation, can be anti-inflammatory instead: for example, TGF-β and IL-10 are both secreted by senescent cells and discourage aggressive T cells and natural killer (NK) cells from destroying them [3, 4]. Similarly, an increase in certain inhibitory immune checkpoints, such as ones involving the programmed death (apoptosis)-related proteins PD-L1 and PD-1, block immune policing in a way that encourages senescence [5]. The author also noted that, on top of an increase in signals that inhibit immune cells from killing other cells (cytotoxicity), the cells themselves appear to be more receptive to these signals with aging.
Five key receptors
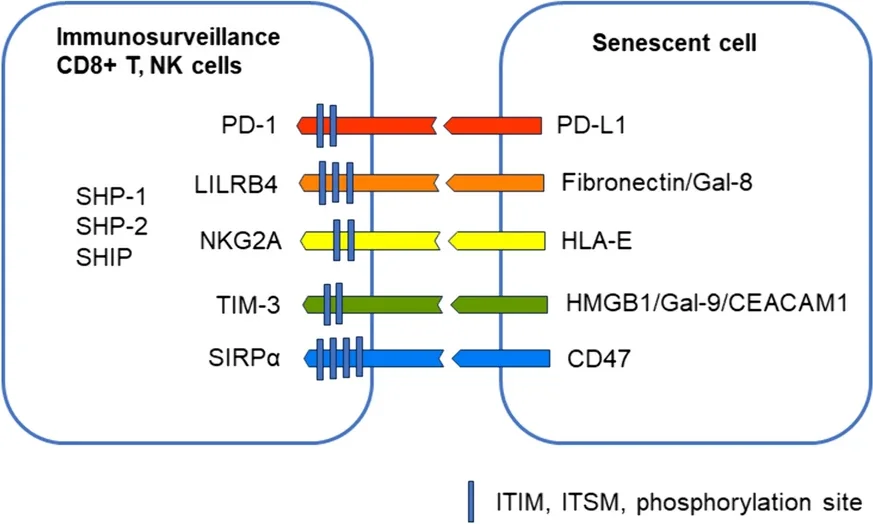
This paper focuses on these five specific receptors, explaining their functions and in what cells they appear.
PD-1 is primarily activated by PD-L1, which is common around the body [6]. While this protein is expressed by healthy cells, it is also strongly expressed by senescent cells and cancer cells, which use it to prevent being attacked by the immune system. While some cancers have managed to avoid attacks on this pathway [7], blocking this pathway may be a viable method of fighting senescence [5].
LILRB4 is also connected to cancer. An experiment in mice found that suppressing this pathway suppressed cancer [8]. However, activating the pathway instead may be a valid method of discouraging autoimmune issues, such as transplanted organ rejection [9]. In aging, LILRB4 is increasingly activated by increased fibronectin, and the literature suggests that it may be protective against conditions such as cardiac hypertrophy [10].
NKG2A is activated by HLA-E, a common protein expressed by cells that have been driven senescent by radiation or chemicals along with non-senescent cells exposed to the SASP [11]. Like other immunosuppressive receptors, cancers express HLA-E to avoid being consumed by the immune system [12].
TIM-3 has been documented to have multiple functions involving the suppression of the immune system. It has been linked to the exhaustion of some T cells while suppressing NK cells and inducing tolerance for transplants and antigens. It has multiple molecules that can activate it, including CEACAM1, which is produced by senescent cells [13].
SIRPα is activated by the CD47 protein, which increases with senescence. Blocking CD47 has been found to fight against atherosclerosis in mice [14]. This may be because CD47 inhibits macrophages from consuming cells that might need to be consumed, such as dying and dysfunctional cells [15]. It is a crucial “self” protein that differentiates the body’s cells from foreign invaders [16].
This is a particularly difficult area to target with drugs. Not only are there multiple potential targets, there is tremendous potential for harm whether they are underactivated or overactivated. By necessity, any research into this area must focus on getting immune cells to attack what they need to attack and leave alone what they need to leave alone.
Literature
[1] Salminen, A. (2021). Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. Journal of Molecular Medicine, 99(11), 1553-1569.
[2] Ruhland, M. K., Loza, A. J., Capietto, A. H., Luo, X., Knolhoff, B. L., Flanagan, K. C., … & Stewart, S. A. (2016). Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nature communications, 7(1), 11762.
[3] Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. L., & Flavell, R. A. (2006). Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol., 24(1), 99-146.
[4] Ouyang, W., Rutz, S., Crellin, N. K., Valdez, P. A., & Hymowitz, S. G. (2011). Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology, 29(1), 71-109.
[5] Wang, T. W., Johmura, Y., Suzuki, N., Omori, S., Migita, T., Yamaguchi, K., … & Nakanishi, M. (2022). Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature, 611(7935), 358-364.
[6] Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., … & Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology, 15(8), 978-990.
[7] Vesely, M. D., Zhang, T., & Chen, L. (2022). Resistance mechanisms to anti-PD cancer immunotherapy. Annual review of immunology, 40(1), 45-74.
[8] Deng, M., Gui, X., Kim, J., Xie, L., Chen, W., Li, Z., … & Zhang, C. C. (2018). LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature, 562(7728), 605-609.
[9] Xiang, Z., Yin, X., Wei, L., Peng, M., Zhu, Q., Lu, X., … & Zou, Y. (2024). LILRB4 Checkpoint for Immunotherapy: Structure, Mechanism and Disease Targets. Biomolecules, 14(2), 187.
[10] Zhou, H., Li, N., Yuan, Y., Jin, Y. G., Wu, Q., Yan, L., … & Tang, Q. Z. (2020). Leukocyte immunoglobulin-like receptor B4 protects against cardiac hypertrophy via SHP-2-dependent inhibition of the NF-κB pathway. Journal of Molecular Medicine, 98, 691-705.
[11] Pereira, B. I., Devine, O. P., Vukmanovic-Stejic, M., Chambers, E. S., Subramanian, P., Patel, N., … & Akbar, A. N. (2019). Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nature communications, 10(1), 2387.
[12] Fisher, J. G., Doyle, A. D., Graham, L. V., Khakoo, S. I., & Blunt, M. D. (2022). Disruption of the NKG2A: HLA-E immune checkpoint axis to enhance NK cell activation against cancer. Vaccines, 10(12), 1993.
[13] Sappino, A. P., Buser, R., Seguin, Q., Fernet, M., Lesne, L., Gumy-Pause, F., … & Mandriota, S. J. (2012). The CEACAM1 tumor suppressor is an ATM and p53-regulated gene required for the induction of cellular senescence by DNA damage. Oncogenesis, 1(4), e7-e7.
[14] Kojima, Y., Volkmer, J. P., McKenna, K., Civelek, M., Lusis, A. J., Miller, C. L., … & Leeper, N. J. (2016). CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature, 536(7614), 86-90.
[15] Logtenberg, M. E., Scheeren, F. A., & Schumacher, T. N. (2020). The CD47-SIRPα immune checkpoint. Immunity, 52(5), 742-752.
[16] Deuse, T., Hu, X., Agbor-Enoh, S., Jang, M. K., Alawi, M., Saygi, C., … & Schrepfer, S. (2021). The SIRPα–CD47 immune checkpoint in NK cells. The Journal of experimental medicine, 218(3).







