NMN: Benefits, Uses, And Side Effects
NMN: Benefits, Uses, And Side Effects
NMN is a naturally occurring molecule and a popular dietary supplement marketed as a NAD+ booster.
What is NMN?
Nicotinamide mononucleotide (NMN) is a naturally occurring molecule present in all species. On the molecular level, it is a ribonucleotide, a basic structural unit of the nucleic acid RNA. It consists of a nicotinamide group, a ribose, and a phosphate group [1].
NMN is a precursor of nicotinamide adenine dinucleotide (NAD+). NAD+ is a molecule that may be useful in slowing down some aspects of aging. It serves many important functions in our cells. This includes things such as electron transport, energy production, cell signaling, and DNA repair [1].
Accumulating evidence suggests that NAD+ levels decline with age, increasing our risk of age-related diseases [2]. This is where NMN comes in and the goal of restoring NAD+ to more youthful levels.
Some researchers suggest if we can restore NAD+ levels, we could slow down aging. This would also probably help delay various age-related diseases. Whether that approach works or not is still an unanswered question, though the animal data is promising [3].
What foods contain NMN?
You can find NMN naturally in foods such as avocado, broccoli, cabbage, cucumber, and edamame. The chart below shows how much NMN is found in some common foods [2].
| Food Type | mg/100 grams |
| Avocado | 0.36 – 1.60 |
| Beef | 0.06 – 0.42 |
| Broccoli | 0.25 – 1.12 |
| Cabbage | 0.0–0.90 |
| Cucumber peel | 0.65 |
| Cucumber seed | 0.56 |
| Edamame | 0.47 – 1.88 |
| Mushroom | 0.0 – 1.01 |
| Shrimp | 0.22 |
| Tomato | 0.26 – 0.30 |
While these kinds of foods are an excellent source of nutrition, dietary NMN supplements are also available. These supplements come in doses of between 100-500 mg, although an optimal dose has yet to be determined in people.
Fasting and caloric restriction also appear to increase NAD+ levels. They also boost the activity of sirtuins, aka the longevity genes. It is suggested that their activity relies on the presence of NAD+. In mice, fasting boosted NAD+ levels and sirtuin activity, and it appears to slow down aging [4].
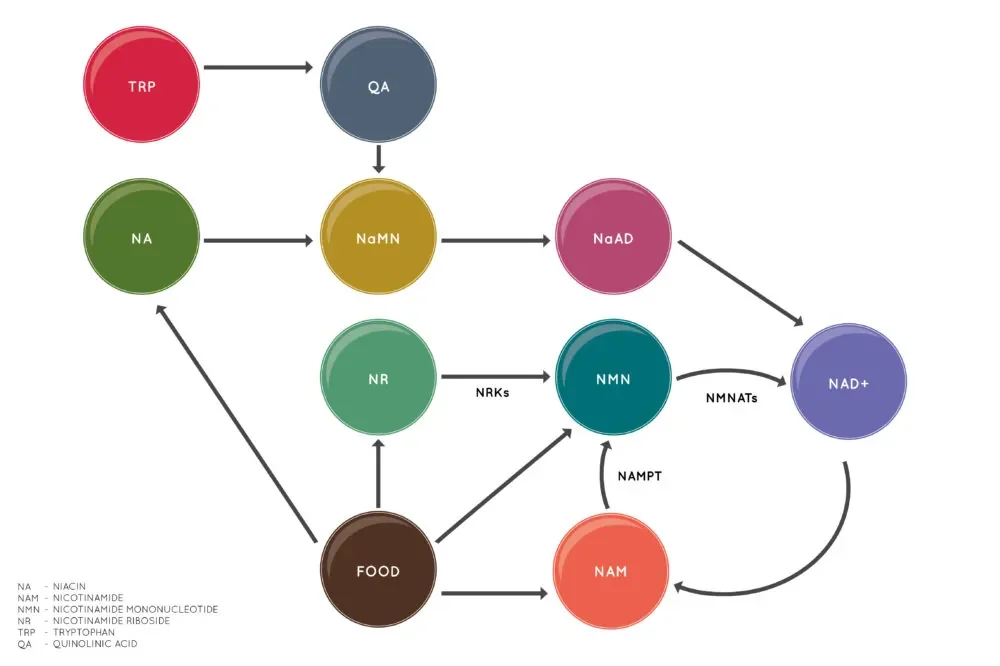
NMN is one of multiple NAD+ precursors
Other NAD+ precursors include nicotinamide riboside (NR) and nicotinic acid (niacin). This diagram shows how these precursors lead to the creation of NAD+ [1].
More recently, reduced nicotinamide mononucleotide (NMNH) has emerged as another NAD+ boosting precursor molecule [5]. However, NMNH is not currently a commonly available dietary supplement, and more research is needed before that happens.
How was NMN discovered?
The history of NMN research is naturally intertwined with the history of NAD+.
In 1963, Chambon, Weill, and Mandel found that NMN gave cells the energy to activate a key nuclear enzyme [6]. This led to the discovery of poly (ADP-ribose) polymerases (PARPs).
This group of proteins helps with many cell processes. These include DNA repair, keeping the genome stable, and programmed cell death. PARPs and their activity are also linked to changes in lifespan in different species.
In 2014, a team of researchers led by Dr. David Sinclair demonstrated that NMN can extend the lifespan of mice [7]. In 2017, researchers used NMN to reverse DNA damage in mice by increasing NAD+ levels [8].
In 2020, researchers used NMN to improve blood flow and neurovascular health in aged mice [9]. It also appeared to reverse some age-related changes to gene expression. Out of 590 genes that differ between young and old animals, NMN treatment changed 204 of them. These genes moved closer to youthful expression levels.
In 2020, a group of researchers demonstrated that treatment with NMN restores neurovascular coupling (NVC) in aged mice [10]. NVC deficiency appears to be a major factor in the age-related decline of cognitive and motor functions.
How is NMN created?
NMN is created in the human body with B vitamins as precursors. The enzyme that makes NMN is known as nicotinamide phosphoribosyltransferase (NAMPT). NAMPT attaches a form of vitamin B3 called nicotinamide to the sugar phosphate 5’-phosphoribosyl-1-pyrophosphate (PRPP).
NAMPT is a rate-limiting enzyme for the production of NAD+. This means that lower levels of NAMPT result in less NMN production and thus decreased NAD+ levels. Also by administering additional NMN, the rate of NAD+ production can be increased to somewhat address this shortfall. It is also possible for NMN to be created from NR by the addition of a phosphate group.
Some researchers suggested that NMN could not enter the cell without first becoming NR. However, in 2019, a new NMN transporter channel was identified showing that it can [11]. They found that the Slc12a8 gene encodes a specific NMN transporter that allows the NMN to enter cells without being converted to NR first. This discovery certainly came as an unpleasant surprise to the manufacturers and distributors of NR supplements.
Human studies of NMN
The bulk of studies on NMN have been carried out on mice and rats. These have shown positive effects on metabolism, liver, skin, muscle, and brain function. Other studies have shown improved bone structure, vascular health, reproduction, immune system function, and lifespan.
Human studies of NMN in people are few in number currently, but there is some limited data of interest.
In 2020 a human clinical trial of NMN showed that it is well tolerated when given as a single dose [12]. The results of a 2021 study suggested that NMN enhances aerobic capacity in amateur runners [13]. While the study did have some limitations, there were some modest improvements.
A 2021 study suggests that NMN improved muscle glucose metabolism in prediabetic women and yielded clinically relevant results [14].
In May 2022, the data from a multicenter NMN clinical trial was published with some modest results [15]. The data suggests NMN increases energy levels and could be interpreted as an anti-aging effect. That said the effect was small in this case.
It is also worth noting that NAD+ levels also increased in the placebo participants not taking NMN. These results require further investigation and a larger study, as this one was fairly small at only 66 people.
NMN and aging
The age-related decline of NAD+ is not just a symptom of aging but a contributing factor to why we age.
As we age, decreased levels of NAD+ impair cellular functions, including energy metabolism and DNA repair [1,2]. This impairment is a key driver of aging and is intimately linked to the development of age-related diseases. NAD+ decline is part of one of the broader reasons we age – deregulated nutrient sensing.
Recent studies show the potential of NMN to elevate NAD+ levels but also enhance mitochondrial function [16]. Mitochondria are essential for energy production, and mitochondrial dysfunction is a reason we age [17]. By boosting NAD+ levels, NMN can enhance mitochondrial activity, thereby potentially alleviating symptoms associated with aging and improving cellular health.
Moreover, the implications of NMN supplementation extend beyond mere energy production. Its role in activating pathways like SIRT1, a NAD+-dependent protein deacetylase, has been linked to various health benefits. These include improved DNA repair, reduced oxidative stress, and enhanced metabolic efficiency. The activation of these pathways highlights the potential of NMN as an anti-aging intervention [16].
As the aging population continues to grow, the significance of NMN in maintaining health becomes ever more pronounced. The ability of NMN to replenish NAD+ levels is a promising avenue to extend lifespan and healthspan.
Cardiovascular health and NMN
Cardiovascular health remains a critical area of concern in modern medicine. Ongoing research is continuously seeking novel approaches to prevent and treat heart-related conditions. In this context, NMN has emerged as a molecule of interest, particularly for its potential benefits in cardiovascular health.
Recent NMN research has shed light on its effects on the heart, especially for conditions that impair its ability to pump blood. A key factor in many heart conditions is mitochondrial dysfunction. This can lead to energy deficits in myocardial cells and contribute to the progression of heart failure.
Mitochondria, being central to energy production in cells, are crucial for maintaining healthy cardiac function. This decline in mitochondrial efficiency is a critical factor in the development of various cardiac diseases [18].
A study focused on heart failure linked to mitochondrial dysfunction revealed the potential of NMN for improving cardiac health. In this study, mice with a cardiomyocyte-specific knockout of the mitochondrial translation factor p32 developed heart failure due to dilated cardiomyopathy. This is a condition characterized by weakened and enlarged heart muscles [18].
This model was crucial in understanding how mitochondrial translation defects lead to mitochondrial dysfunction and a decrease in NAD+. This decrease impairs lysosomal acidification and autophagy, which are essential for cellular waste disposal and recycling [19].
In this scenario, NMN administration showcased a remarkable ability to compensate for the decreased NAD+ levels. Supplementation with NMN led to reduced damaged lysosomes and improved autophagy. This thereby ameliorated heart failure and extended lifespan in the mice. Interestingly, the study highlighted that the ameliorative effects of NMN supplementation were more pronounced on lysosomal function rather than directly on mitochondrial function.
It was observed that lysosomal damage due to mitochondrial dysfunction could induce ferroptosis. This is a form of cell death due to iron accumulation and lipid peroxidation. NMN’s role in preventing ferroptosis, may be a way through which NMN supplementation could support cardiac health [19].
The implications of this study are profound for cardiovascular medicine. NMN supplementation offers a potential strategy for mitigating the effects of heart failure while addressing underlying mitochondrial and lysosomal dysfunctions. This suggests that focusing on cellular health could potentially improve organ function and help prevent age-related diseases.
A recent study investigated NMN’s effects on atherosclerosis, a major contributor to cardiovascular disease [20]. This study was conducted on ApoE-/- mice, a model widely used in atherosclerosis research. It revealed that NMN significantly reduced atherosclerotic plaque by 36% and the necrotic core by 48%. Furthermore, NMN improved lesion composition by decreasing lipid area by 43% and increasing collagen content by 51%.
These findings suggest NMN has the potential to reduce key risk factors for cardiovascular diseases. It also highlights its role in enhancing vascular cell function, which is vital for preventing and treating atherosclerosis.
As NMN research continues, its role in cardiovascular health offers new hope for people affected by heart diseases. It also highlights the interconnected nature of aging, cellular health, and organ function.
NMN, inflammation, and immune response
The same study also brought to light its potential in managing chronic inflammatory diseases [20]. NMN administration was observed to lower serum levels of malondialdehyde (MDA), a marker of oxidative stress, and increase activities of antioxidant enzymes. Notably, it reduced the expression of pro-inflammatory cytokines while enhancing the expression of anti-inflammatory factors in aortic tissues.
These results suggest that NMN’s capacity to mitigate oxidative stress and modulate inflammatory responses could be pivotal in addressing various chronic inflammatory conditions [20].
Another study looked at human cells, like lung microvascular endothelial cells and coronary artery endothelial cells (HCAECs). It found that NMN supplementation can reduce the inflammation caused by poly(I:C). Poly(I:C) is a synthetic analog of double-stranded RNA.
This effect is partly due to the downregulation of key inflammatory mediators. These include IL6 and PARP family members when exposed to NMN. Such findings illustrate NMN’s capacity to influence inflammatory pathways at the molecular level.
The implications of these findings are substantial. For chronic inflammation, which is often a silent and insidious process contributing to various age-related diseases. NMN’s potential to modulate immune responses and reduce inflammatory markers can be a game-changer.
This could lead to novel approaches in treating conditions in which chronic inflammation is a key component. Conditions such as in certain autoimmune diseases, metabolic syndrome, and even some neurodegenerative disorders.
Relating to acute inflammation, typically a rapid and intense response to injury or infection, NMN also shows promise. The ability to control and limit acute inflammatory responses could enhance recovery and healing processes. Meaning it might potentially reduce the risk of complications such as tissue damage and scarring [22].
Cognitive function and metabolic health
The exploration of NMN in the realms of cognitive function and metabolic health has yielded promising insights. Cognitive function, which encompasses memory, attention, and other mental capabilities, often deteriorates with age.
Metabolic health is vitally important for the body’s energy balance and overall well-being. However, it can be affected by lifestyle choices and aging. NMN’s role in these areas offers a glimpse into its broader therapeutic potential.
Recent studies have indicated that NMN can have a significant impact on cognitive function. A study focused on the combined effects of NMN and sugars called neoagarooligosaccharides (NAOS). Researchers explored how these compounds can improve brain health and reduce cell damage and inflammation [23].
The study used two models. One was a mouse breed called SAMP8, which ages quickly. The other was human lung cells. These cells were treated with hydrogen peroxide (H2O2) to simulate stress.
The combined treatment of NMN and NAOS was found to significantly improve learning and memory in the mice. This was seen in the passive avoidance and the Morris water maze tests. These tests are standard methods to assess cognitive abilities in animals.
This combination increased proteins that are essential for maintaining healthy mitochondria in the brains of these mice. Specifically, the levels of OPA1 and mitofusin 2, two proteins important for mitochondrial function, were increased significantly.
The treatment with NMN and NAOS helped reduce cell death (apoptosis) in aging mice and stressed human lung cells. This effect was evidenced by the decrease in certain proteins (Bax and caspase-3) that are markers of cell death. Also by a reduction in the activity of the cellular pathway (p53/p21/p16) known to be involved in this process.
In addition, this combination also lessened inflammation by downregulating a specific inflammatory pathway (TLR4/MyD88/NF-κB) in the cells. Improved mitochondrial function in neural cells ensures better energy supply. This may help in the maintenance and repair of neuronal networks, thereby supporting cognitive health.
In another study, researchers focused on a condition known as postoperative cognitive dysfunction (POCD). This is a condition in which patients experience a decline in mental functions like memory and concentration after surgery. This is often linked to general anesthesia.
A key factor in this condition is oxidative stress. This stress happens when the body cannot detoxify harmful substances properly to detoxify harmful substances.
Past studies have shown that lower levels of NAD+ and sirtuin 1 (SIRT1) can raise oxidative stress levels. The study looked at whether taking NMN before surgery could help brain function and reduce oxidative stress in POCD.
To simulate POCD, researchers used a common mouse breed called C57BL/6J. They caused cognitive impairment by using isoflurane anesthesia for 6 hours. Isoflurane is a type of anesthesia often used in surgeries.
The mice were given NMN injections for 7 days before being exposed to anesthesia. To assess the effects, the study measured the levels of oxidative stress and evaluated cognitive functions.
They used flow cytometry and specific test kits to measure oxidative stress. Additionally, they conducted the fear conditioning test and the Y-maze test. These are both standard methods to assess memory and learning in animals.
The results showed that the mice undergoing anesthesia experienced cognitive difficulties and higher levels of oxidative stress. There was also a decrease in NAD+ and SIRT1 levels in the hippocampus, a crucial brain region for memory and learning.
The mice that received NMN before surgery showed less decline in NAD+. They also had lower oxidative stress and fewer cognitive problems after surgery.
The study suggests that taking NMN before surgery may help protect cognitive function. It does this by affecting the NAD+-SIRT1 signaling pathway. This pathway is important for managing cell health and stress responses in the body. This suggests that NMN could be a potential way to reduce brain damage in patients undergoing surgeries [24].
Bioavailability and formulation advances
There have been some attempts to increase the efficacy of NMN by increasing its bioavailability. Bioavailability is an important factor in how well a dietary supplement works. It determines how much of the compound gets into the bloodstream and is available for the body to use.
Innovations in NMN formulation, such as by combining it with hydroxyapatite (NMN-HAP), represent a breakthrough in addressing absorption and utilization challenges. This wet chemical precipitation and physical adsorption method can significantly enhance the encapsulation efficiency and drug loading capacity of NMN.
These NMN-HAP nanoparticles are rod-shaped and about 50 nanometers in size. They show a great improvement in releasing NMN in a controlled way compared to its free form. This approach ensures a more steady and sustained release of NMN into the body, potentially maximizing its biological impact.
In vivo studies of NMN-HAP have shown good results. It has a longer circulation time and better bioavailability than free NMN. The formulation increased the plasma levels of NMN, NAD+, and nicotinamide riboside (NR), suggesting a more efficient delivery system.
The tissue-specific distribution shows a large buildup of NMN, NAD+, and NR in the brain and liver. This highlights how effective this advanced formulation is [25].
Another study looked at how to make lycopene (LYC) and NMN more stable and effective. They did this by putting them into small, porous gel-like particles called microgels [26]. This came after they had already placed LYC into tiny fat-like particles known as liposomes.
Next they tested how well the microgels held LYC and NMN. The tests showed that the microgels were very effective. They contained high levels of LYC and NMN, at 99.11% and 68.98%, respectively. The microgels were stable and released their contents well.
Then they examined if the microgels could protect against acute liver injury caused by a substance called lipopolysaccharide (LPS). Mice were given these microgels for 28 days before being exposed to LPS. The microgels with LYC and NMN significantly reduced liver injury from LPS. They also lowered inflammation and oxidative stress.
The study found that LYC and NMN in the microgels targeted a specific part of the cell called the TLR4/MD2 complex. They also regulated certain microRNAs linked to this receptor. These microRNAs play a role in controlling inflammation.
This action helped inhibit a specific signaling pathway in the body (TLR4/NF-κB) known to be involved in inflammation. The microgels also increased the presence of beneficial gut bacteria that produce short-chain fatty acids. Plus they reduced the presence of harmful bacteria.
The study concluded that LYC and NMN, delivered through these microgels, can help protect the liver from injury caused by LPS. They do this by reducing oxidative stress, inflammation, and by helping regulate the balance of bacteria in the gut. These new methods improve how NMN is delivered and absorbed and may positively affect health.
Using biocompatible materials like hydroxyapatite in NMN-HAP shows a strong focus on safety and effectiveness of NMN supplements. As research continues to progress even more effective and targeted NMN-based therapies may arrive. This could potentially change the approach to health supplementation and the management of age-related conditions.
The future of NMN research
Human data seems promising, but there still is a lack of a full understanding. But, this hasn’t stopped NMN from being enthusiastically marketed as a dietary supplement and a NAD+ booster. It’s a popular supplement despite the lack of strong human evidence.
However, the high cost may be prohibitive to some people seeking the fountain of youth. After all, to be truly effective in slowing down aging, a supplement needs to work and be affordable. Currently, the price of NMN and NR is beyond the reach of the average person.
However, we have not yet been able to confirm that NMN supplements actually slow down aging. Fortunately, there are more human trials underway that will hopefully tell us how useful NMN is in this respect.
Nonetheless, recent clinical trials have predominantly affirmed the safety and tolerability of NMN supplementation in humans. For example, studies have shown that taking NMN daily in different doses is safe and does not cause major side effects.
These trials have focused on different areas. Some looked at how NMN affects blood NAD+ levels. Others assessed wider health outcomes. These include cognitive function, metabolic health, and heart health.
A key finding from these trials is the significant rise in plasma NAD+ levels after NMN supplementation. Some studies show that raising NAD+ levels can improve various markers of health. This includes better physical performance and stabilization of blood biological age. People also report better overall health assessment scores.
These results suggest that NMN supports cellular health. It may also improve overall well-being and slow down some aspects of aging [27].
However, one interesting aspect that has emerged from these trials is the variability in individual responses to NMN supplementation. This variability suggests a nuanced picture of NMN’s effects. It suggests that personal factors like genetics, lifestyle, and health can affect how a person responds to NMN.
This underscores the importance of personalized approaches in NMN supplementation. It suggests that future research and applications need to tailor NMN dosages and regimens to individual needs and contexts.
The safety profile of NMN, as evidenced by these trials, is particularly encouraging. It reinforces the potential of NMN as a supplement for long-term use. The lack of significant adverse effects in trial participants is a testament to NMN’s suitability for widespread use [27].
NMN side effects
The data on long-term use of NMN in humans is not complete. However, many people take it as a dietary supplement. There are few negative reports about its use. However, more studies focused on long-term safety and efficacy should be conducted.
Early adopters who take NMN supplements are essentially self-experimenting. Anyone experiencing adverse side effects should cease consumption and consult a medical professional.
Disclaimer
This article is only a summary and is not intended as an exhaustive guide. The article is based on the interpretation of research data, which is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you.
We do not endorse supplement use nor any product or supplement vendor. All information presented here is for scientific interest.
Literature
[1] Nicotinamide Mononucleotide | C11H15N2O8P | CID 14180
[2] Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab 2016, 24, 795–806
[3] Kim, L.J.; Chalmers, T.J.; Madawala, R.; Smith, G.C.; Li, C.; Das, A.; Poon, E.W.K.; Wang, J.; Tucker, S.P.; Sinclair, D.A.; et al. Host–Microbiome Interactions in Nicotinamide Mononucleotide (NMN) Deamidation. FEBS Lett 2023, 597, 2196–2220
[4] Hayashida, S.; Arimoto, A.; Kuramoto, Y.; Kozako, T.; Honda, S.I.; Shimeno, H.; Soeda, S. Fasting Promotes the Expression of SIRT1, an NAD+-Dependent Protein Deacetylase, via Activation of PPARa in Mice. Mol Cell Biochem 2010, 339, 285–292
[5] Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Møller, N.; Brenner, C.; Treebak, J.T.; et al. A Randomized Placebo-Controlled Clinical Trial of Nicotinamide Riboside in Obese Men: Safety, Insulin-Sensitivity, and Lipid-Mobilizing Effects. Am J Clin Nutr 2018, 108, 343–353
[6] Chambon, P.; Weill, J.D.; Mandel, P. Nicotinamide Mononucleotide Activation of New DNA-Dependent Polyadenylic Acid Synthesizing Nuclear Enzyme. Biochem Biophys Res Commun 1963, 11, 39–43
[7] North, B.J.; Rosenberg, M.A.; Jeganathan, K.B.; Hafner, A. V; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; Wu, L.E.; Sauve, A.A.; et al. SIRT 2 Induces the Checkpoint Kinase BubR1 to Increase Lifespan. EMBO J 2014, 33, 1438–1453
[8] Li, J.; Bonkowski, M.S.; Moniot, S.; Zhang, D.; Hubbard, B.P.; Ling, A.J.Y.; Rajman, L.A.; Qin, B.; Lou, Z.; Gorbunova, V.; et al. A Conserved NAD+ Binding Pocket That Regulates Protein-Protein Interactions during Aging. Science 2017, 355, 1312–1317
[9] Kiss, T.; Nyúl-Tóth, Á.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide Mononucleotide (NMN) Supplementation Promotes Neurovascular Rejuvenation in Aged Mice: Transcriptional Footprint of SIRT1 Activation, Mitochondrial Protection, Anti-Inflammatory, and Anti-Apoptotic Effects. Geroscience 2020, 42, 527–546
[10] Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Süle, Z.; et al. Nicotinamide Mononucleotide (NMN) Supplementation Rescues Cerebromicrovascular Endothelial Function and Neurovascular Coupling Responses and Improves Cognitive Function in Aged Mice. Redox Biol 2019, 24, 101192
[11] Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E.; et al. Slc12a8 Is a Nicotinamide Mononucleotide Transporter. Nat Metab 2019, 1, 47–57
[12] Irie, J.; Inagaki, E.; Fujita, M.; Nakaya, H.; Mitsuishi, M.; Yamaguchi, S.; Yamashita, K.; Shigaki, S.; Ono, T.; Yukioka, H.; et al. Effect of Oral Administration of Nicotinamide Mononucleotide on Clinical Parameters and Nicotinamide Metabolite Levels in Healthy Japanese Men. Endocr J 2020, 67, 153–160
[13] Liao, B.; Zhao, Y.; Wang, D.; Zhang, X.; Hao, X.; Hu, M. Nicotinamide Mononucleotide Supplementation Enhances Aerobic Capacity in Amateur Runners: A Randomized, Double-Blind Study. J Int Soc Sports Nutr 2021, 18
[14] Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.I.; et al. Nicotinamide Mononucleotide Increases Muscle Insulin Sensitivity in Prediabetic Women. Science 2021, 372, 1224–1229
[15] Huang, H. A Multicentre, Randomised, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation in Middle Aged and Older Adults. Frontiers in aging 2022, 3
[16] He, S.; Jiang, X.; Yang, J.; Wu, Y.; Shi, J.; Wu, X.; Du, S.; Zhang, Y.; Gong, L.; Dong, S.; et al. Nicotinamide Mononucleotide Alleviates Endotoxin-Induced Acute Lung Injury by Modulating Macrophage Polarization via the SIRT1/NF-?B Pathway. Pharm Biol 2024, 62, 22–32
[17] López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194
[18] Wu, Y.; Pei, Z.; Qu, P. NAD+-A Hub of Energy Metabolism in Heart Failure. Int J Med Sci 2024, 21, 369–375
[19] Yagi, M.; Do, Y.; Hirai, H.; Miki, K.; Toshima, T.; Fukahori, Y.; Setoyama, D.; Abe, C.; Nabeshima, Y.I.; Kang, D.; et al. Improving Lysosomal Ferroptosis with NMN Administration Protects against Heart Failure. Life Sci Alliance 2023, 6
[20] Wang, Z.; Zhou, S.; Hao, Y.; Xu, T.; An, P.; Luo, Y.; Luo, J. Nicotinamide Mononucleotide Protects against High-Fat-Diet-Induced Atherosclerosis in Mice and Dampens Aortic Inflammation and Oxidative Stress. J Funct Foods 2024, 112
[21] Sano, H.; Kratz, A.; Nishino, T.; Imamura, H.; Yoshida, Y.; Shimizu, N.; Kitano, H.; Yachie, A. Nicotinamide Mononucleotide (NMN) Alleviates the Poly(I:C)-Induced Inflammatory Response in Human Primary Cell Cultures. Scientific Reports 2023 13:1 2023, 13, 1–12
[22] Liang, Y.; Li, M.; Tang, Y.; Yang, J.; Wang, J.; Zhu, Y.; Liang, H.; Lin, Q.; Cheng, Y.; Yang, X.; et al. Temperature-Sensitive Hydrogel Dressing Loaded with Nicotinamide Mononucleotide Accelerating Wound Healing in Diabetic Mice. Biomedicine & Pharmacotherapy 2023, 167, 115431
[23] Li, T.; Li, Y.; Yan, Q.; Jiang, Z.; Yang, S. Co-Treatment of Nicotinamide Mononucleotide and Neoagarooligosaccharide Mitigates Aging-Induced Cognitive Impairment by Promoting Mitochondrial Dynamics. J Funct Foods 2024, 112
[24] Xu, J.; Chen, X.; Liu, S.; Wei, Z.; Xu, M.; Jiang, L.; Han, X.; Peng, L.; Gu, X.; Xia, T. Nicotinamide Mononucleotide Pretreatment Improves Long-Term Isoflurane Anesthesia-Induced Cognitive Impairment in Mice. Behavioural Brain Research 2024, 458, 114738
[25] Zhang, D.; Yau, L.-F.; Bai, L.-B.; Tong, T.-T.; Cao, K.-Y.; Yan, T.-M.; Zeng, L.; Jiang, Z.-H. Hydroxyapatite-Based Nano-Drug Delivery System for Nicotinamide Mononucleotide (NMN): Significantly Enhancing NMN Bioavailability and Replenishing in Vivo Nicotinamide Adenine Dinucleotide (NAD+) Levels. Journal of Pharmacy and Pharmacology 2023, 75, 1569–1580
[26] Ge, J.; Ye, L.; Cheng, M.; Xu, W.; Chen, Z.; Guan, F. Preparation of Microgels Loaded with Lycopene/NMN and Their Protective Mechanism against Acute Liver Injury. Food Funct 2023
[27] Song, Q.; Zhou, X.; Xu, K.; Liu, S.; Zhu, X.; Yang, J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update. Advances in Nutrition 2023, 14, 1416–1435